Soil organic matter is a mixture of carbon compounds from decomposing plants and organisms. This carbon directly influences the life-supporting services provided by soils, including the purification and retention of water and the regulation of atmospheric carbon dioxide.
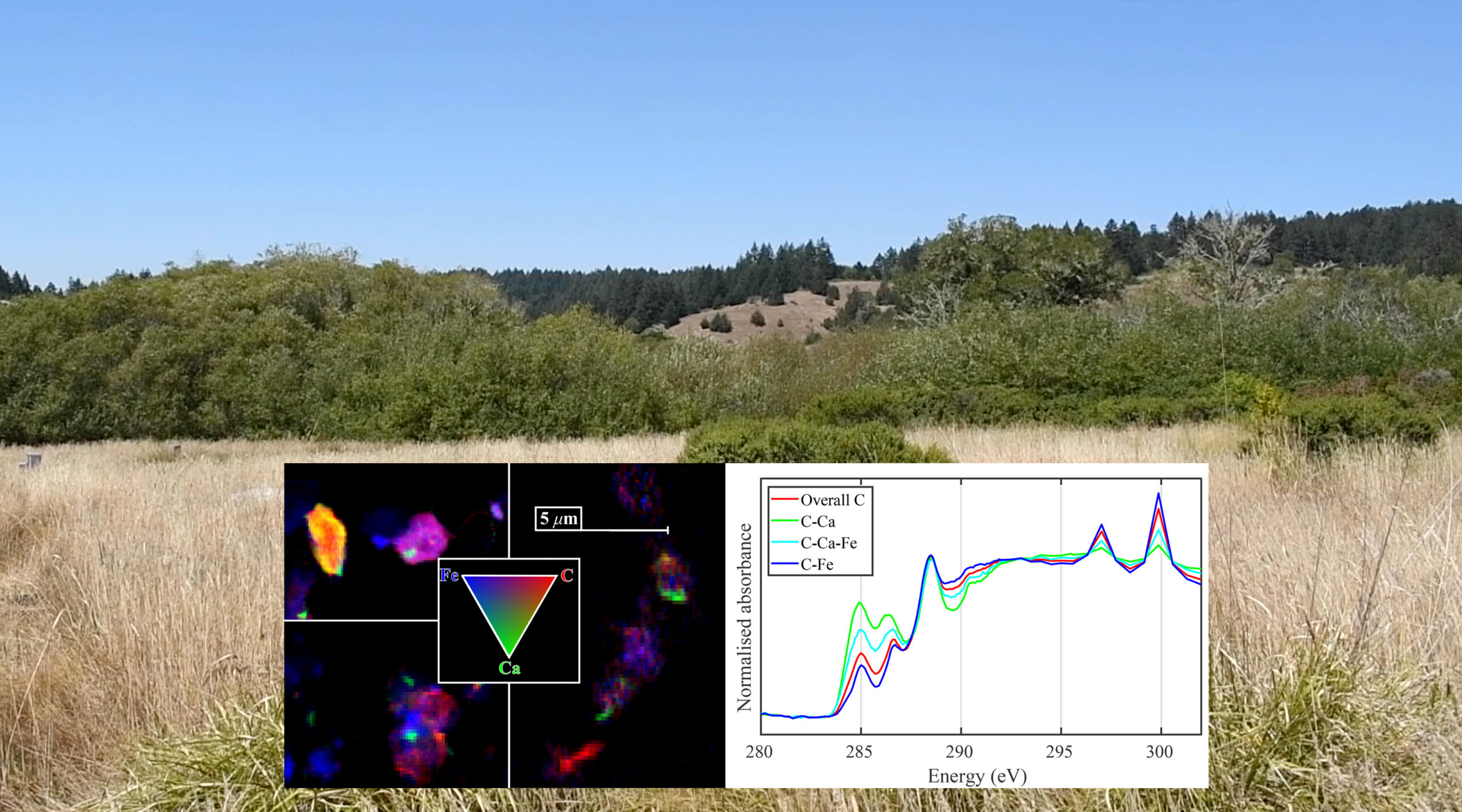
Previous studies have shown that the amount of organic carbon in soils from a range of ecosystems can be linked to calcium. This relationship is thought to arise because positively charged calcium (Ca2+) can bind negatively charged carbon to mineral surfaces and contributes to soil aggregation, which tends to stabilize and preserve organic matter. However, most studies have focused on high-pH (alkaline) soils, overlooking potential interactions between calcium and carbon in acidic soils.
“This work highlights an important and previously understudied association between soil organic matter and calcium in the acidic soils of California,” said first author Mike Rowley, a recent postdoc in the Belowground Biogeochemistry team at Berkeley Lab and now a biogeochemist at the University of Zurich. “The interactions between soil organic matter and minerals or metals is important for the global carbon cycle, as it can lead to the retention and accumulation of carbon in soils.”
An international team of researchers analyzed soils with a range of acidic pH values, collected from coastal grasslands at Point Reyes National Seashore in California. At the Advanced Light Source (ALS), they used scanning transmission x-ray microscopy (STXM) at Beamline 5.3.2.2 to characterize the chemical state of the organic carbon in the samples.
The data showed that the carbon associated with calcium has a unique signature relative to that of carbon associated with iron (typically thought to play a large role in carbon stabilization of acidic soils). The calcium-associated carbon had lower proportions of O-alkyl carbon (polysaccharides, alcohols, hydroxylated and ether-linked carbon) and higher relative abundances of aromatic (ring shaped) and phenolic (OH-bonded ring-shaped) carbon. This implies that the calcium-associated carbon was more plant-like in nature than iron-associated carbon.
The results highlight the importance of calcium–carbon interactions in acidic grassland soils and provide a conceptual model of its contribution to the preservation and accumulation of partially transformed, plant-like carbon in the acidic grassland soils of Northern California.
M.C. Rowley, P.S. Nico, S.E. Bone, M.A. Marcus, E.F. Pegoraro, C. Castanha, K. Kang, A. Bhattacharyya, M.S. Torn, and J. Peña, “Association between soil organic carbon and calcium in acidic grassland soils from Point Reyes National Seashore, CA,” Biogeochemistry 165, 91 (2023), doi:10.1007/s10533-023-01059-2.