SCIENTIFIC ACHIEVEMENT
Using the Advanced Light Source (ALS), researchers clarified the mechanism—an unusual intermediate state—that accelerates the transformation of nanocrystals into a superlattice with fewer defects using a two-step, instead of a one-step, process.
SIGNIFICANCE AND IMPACT
The new approach, which can control the development of materials with desired characteristics, could be applied to electronics, energy storage, and even medicine.
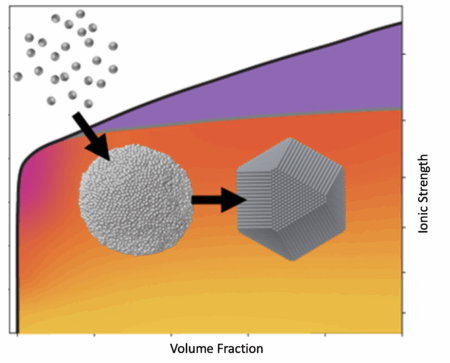
The art of crystallization
In nature, atoms align and form organized, repeating, three-dimensional solid structures called lattices. Researchers have long sought to mimic this process in the lab with nanocrystal building blocks that are larger and more varied than atoms to create new superlattice materials with desirable characteristics. These new materials could be used in electronics, energy storage, and even medicine.
Traditional techniques favor a one-step crystallization process using a colloidal phase that forces particles into the superlattice by increasing their density. A two-step pathway, however, has been shown to increase the rate of crystallization even as the process is driven harder, but the underlying mechanism behind this approach remains unclear.
A multi-institutional team of researchers led by UC Berkeley collaborated with scientists from the ALS at Lawrence Berkeley National Laboratory (Berkeley Lab) to reveal the details of the pathways to efficiently assemble a superlattice of nanocrystals that is more effective at conducting electricity.
Characterizing the mechanism of self-assembly
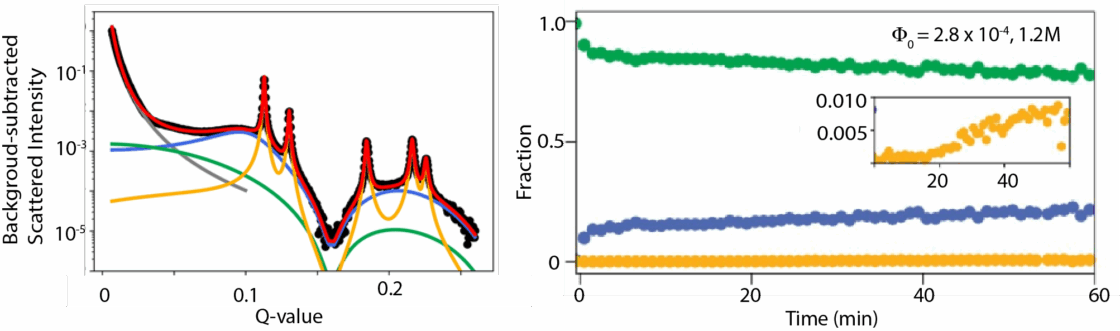
In the study, the researchers used electrostatics to form complex building blocks that self-assemble through a two-step crystallization process into a superlattice with fewer defects than through a one-step process. The team conducted small-angle x-ray scattering (SAXS) experiments using an anoxic, gas-tight reactor at Berkeley Lab’s Beamline 7.3.3 at the ALS. This setup enabled in situ, real-time tracking of scattering patterns as nanocrystal suspensions transformed into superlattices.
To modulate interparticle interactions, the researchers injected salt solutions of varying concentrations into the nanocrystal suspension to tune the interaction strength and control the self-assembly process. The high synchrotron flux provided the sensitivity to resolve phase evolution with excellent signal-to-noise ratio, allowing clear distinction among coexisting colloidal, liquid, and superlattice phases as the system evolved.
The researchers fit the SAXS data to a new model and quantitatively tracked the time-dependent evolution of each phase, revealing the interplay between colloidal dispersions, metastable liquid intermediates, and emerging superlattice order. These insights offered a mechanistic understanding of how nanoscale interactions direct crystallization pathways and assembly kinetics. The ALS experiments were further complemented by measurements at SLAC National Laboratory’s Stanford Synchrotron Radiation Lightsource.
A faster system with fewer defects
The results revealed the mechanism that drives two-step crystallization. At the beginning of the experiment, the nanocrystals condensed into a dense, liquid-like state that the researchers describe as a temporary metastable liquid phase. This intermediary phase gave the nanocrystals the time and space to assemble into superlattices, resulting in fewer defects. The researchers also determined that it was possible to adjust the nanocrystal assembly time—from seconds to hours—by varying the salt concentration in the solution.
The bottom-up assembly of a superlattice illustrated the ability to control the chemistry of the reactants to create materials with desired features. Future projects could further improve this approach by designing even more sophisticated protocols for this two-step, self-assembly mechanism. Ultimately, this work could yield intricate materials to create complex systems for catalysis, drug delivery, and energy storage.
Contacts: Naomi Ginsberg and Chenhui Zhu
Funding: US Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES), Materials Sciences and Engineering Division; University of Chicago Materials Research Science and Engineering Center, supported by the National Science Foundation (NSF); NSF Graduate Research Fellowship program; National Defense Science and Engineering Graduate Fellowship program, Arnold and Mabel Beckman Foundation; Kavli Energy NanoScience Institute; Kwanjeong Educational Foundation; Alfred P. Sloan Research Fellowship, the David and Lucile Packard Foundation Fellowships for Science and Engineering; and Camille and Henry Dreyfus Teacher-Scholar Awards. Operations of the ALS and the Stanford Synchrotron Radiation Lightsource at SLAC National Accelerator Laboratory are supported by DOE BES.
Researchers: C.P.N. Tanner, V.R.K. Wall, A. Das, J.K. Utterback, L.M. Hamerlynck, J.G. Raybin, R.B. Wai, J.A. Tan, M. Gababa, and D.T. Limmer (University of California, Berkeley); J. Portner and A. Jeong (University of Chicago); M.J. Hurley, N. Leonard, and S.W. Teitelbaum (Arizona State University); C. Zhu, E. Schaible (ALS); C.J. Tassone (Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory); D.V. Talapin (University of Chicago and Argonne National Laboratory); and Naomi S. Ginsberg (University of California, Berkeley, Berkeley Lab, and STROBE)
Publication: C.P.N. Tanner, V.R.K. Wall, J. Portner, A. Jeong, A. Das, J.K. Utterback, L.M. Hamerlynck, J.G. Raybin, M.J. Hurley, N. Leonard, R.B. Wai, J.A. Tan, M. Gababa, C. Zhu, E. Schaible, C.J. Tassone, D.T. Limmer, S.W. Teitelbaum, D.V. Talapin, and N.S. Ginsberg, “Enhancing nanoscale charged colloid crystallization near a metastable liquid binodal,” Nat. Phys, 21, 1594–1602 (2025), doi: 10.1038/s41567-025-02996-5.
ALS SCIENCE HIGHLIGHT #534
10/25