The heterogeneous catalysts used in most chemical processes typically consist of nanoscale metal or metal oxide particles dispersed on high-surface-area supports. While these particles are the active elements of the catalyst, the overall performance depends not only on their size and composition but also on their multiple interactions with the support, reactants, and products. Probing this chemical soup in real time under realistic reaction conditions is such a tall order that in some cases even the catalytically active chemical species is not known. A Dutch team working at the ALS has combined scanning transmission x-ray microscopy with a reaction chamber adapted from electron microscopy to identify the chemical species present for an iron-based Fischer–Tropsch synthesis catalyst and to image their distribution on the nanoscale. When developed further, this new tool may give chemists the ability to design and tailor catalysts for maximum selectivity and efficiency in a wide range of chemical processes.
In the Fischer–Tropsch process, synthesis gas, a mixture of CO and H2, is converted through a surface polymerization reaction into liquid hydrocarbons of various forms that can then be used in the production of high-purity chemicals and transportation fuels without the need for crude oil. The most common catalysts are based on iron and cobalt. For its in-situ soft x-ray scanning transmission microscopy (STXM) study at ALS Beamline 11.0.2, the Dutch team used an iron-based catalyst consisting of an iron oxide phase dispersed on silicon oxide (SiO2), with copper oxide and potassium oxide promoters added to improve its selectivity, activity, and stability. The lack of in-situ data under reaction conditions makes it difficult to control phase transformations in the complex iron–oxygen–carbon system, which can be important in maintaining catalyst performance.

Use of STXM to study this catalyst under reaction conditions solves the nanoscale part of the problem but suffers from the transmission-limiting high gas pressures (one atmosphere or higher) typically encountered. To obtain high-quality data, the researchers adapted a “nanoreactor” originally designed for in-situ electron microscopy. The small distance through which the soft x-rays traveled in the reaction chamber together with thin silicon nitride windows allowed measurements at atmospheric pressure, and an embedded platinum spiral heater produced temperatures up to 500 °C.
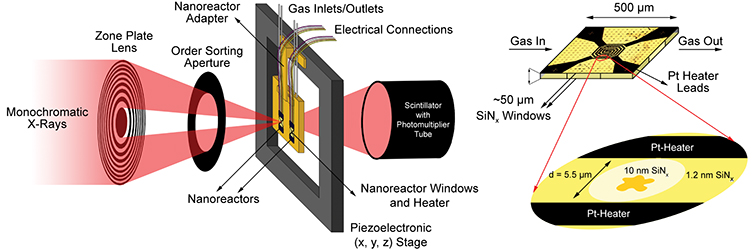
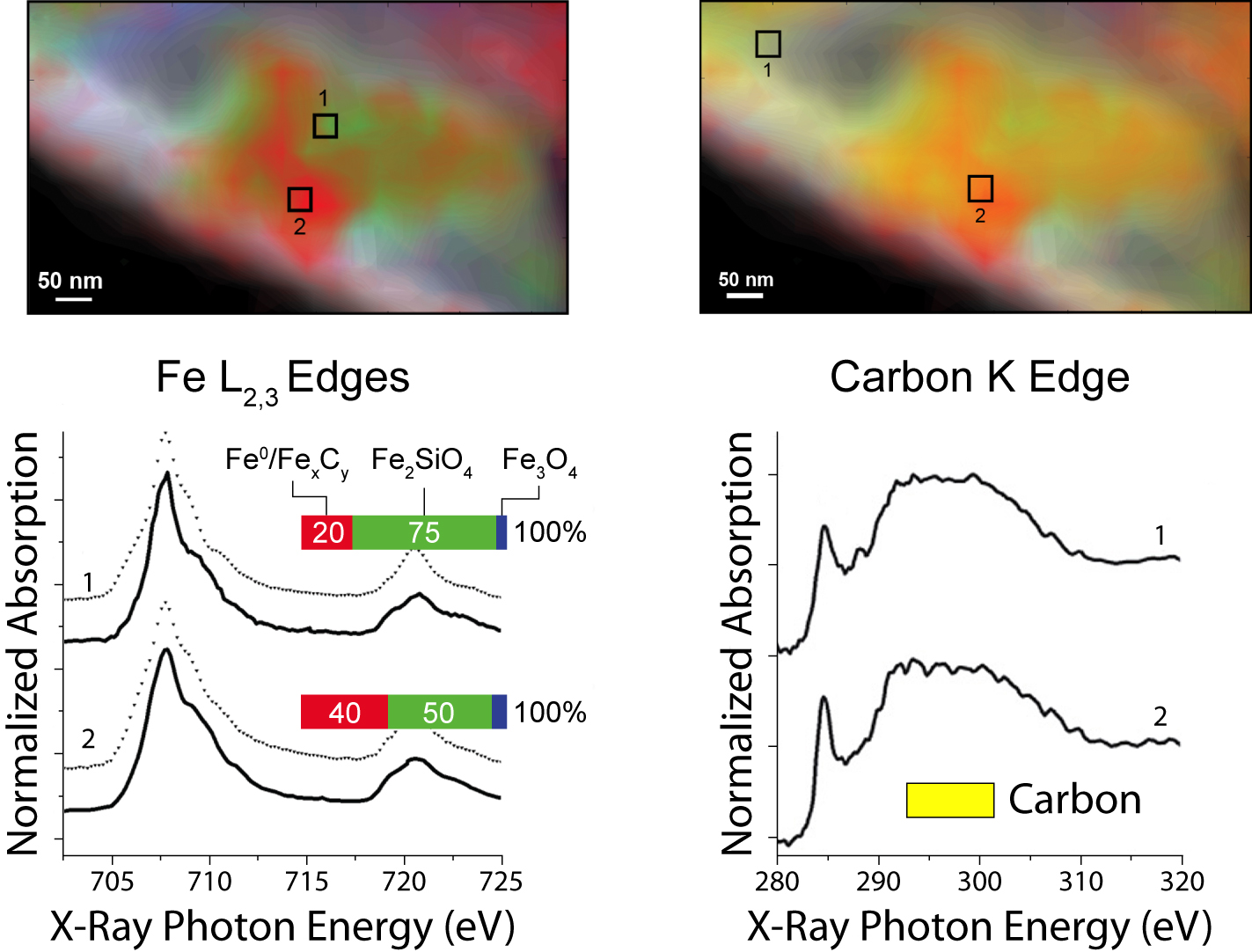
The experiment itself comprised three series of STXM measurements with a spatial resolution of 40 nm: first, in initial characterization at room temperature in helium; then, after two hours at 350 °C in reducing hydrogen gas; and lastly, after a further 4 hours at 250 °C in synthesis gas. To identify the valence and coordination of iron species, spectra were taken for each pixel at the iron L3 and L2 edges; for oxygen-containing species, at the oxygen K edge; and for carbon-containing species, at the carbon K edge. The measurements generated chemical contour maps for the species present. Quantitative analysis of the spectra yielded calculated proportions for the various phases present at each point.
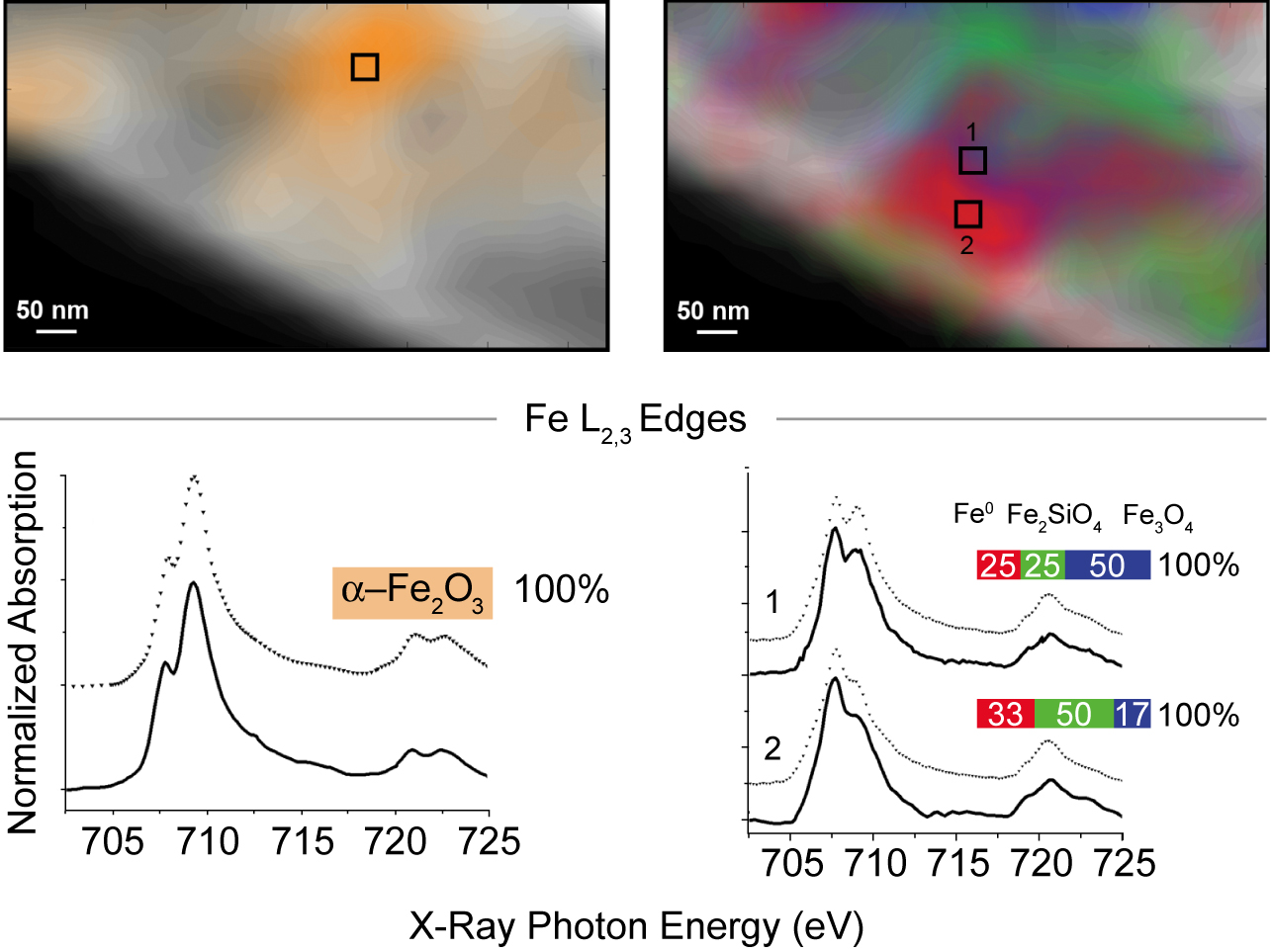
In brief, initially there was a heterogeneous distribution of predominantly α-Fe2O3 (the catalyst precursor) and SiO2 (the support) with no evidence of carbides. After the reducing treatment in hydrogen, significant changes included the appearance of a heterogeneous distribution of Fe3O4, FeSiO4, and an Fe0 species. During catalysis, the proportion of FeSiO4 grew at the expense of Fe3O4. Carbon K-edge spectra indicate the preferential presence of carbon with the Fe0, suggesting the formation of iron carbides, a finding consistent with prior knowledge that during a reaction, iron oxide and metallic iron (α-Fe) usually coexist, with the iron phases largely converted into iron carbide. These measurements demonstrate that STXM can provide details about the morphology and composition of complex catalytic systems under realistic conditions.
Contacts: Frank de Groot and Bert Weckhuysen
Research conducted by E. de Smit, I. Swart, C. Morin, B.M. Weckhuysen, and F.M.F. de Groot (Utrecht University, The Netherlands); J.F. Creemer, G.H. Hoveling, P.J. Kooyman, and H.W. Zandbergen (Delft University of Technology, The Netherlands); and M.K. Gilles and T. Tyliszczak (ALS).
Research funding: Dutch National Science Foundation, the Netherlands Research School Combination on Catalysis, Shell Global Solutions, and the U.S. Department of Energy, Office of Basic Energy Sciences (BES). Operation of the ALS is supported by BES.
Publication about this research: E. de Smit, I. Swart, J.F. Creemer, G.H. Hoveling, M.K. Gilles, T. Tyliszczak, P.J. Kooyman, H.W. Zandbergen, C. Morin, B.M. Weckhuysen, and F.M.F. de Groot, “Nanoscale chemical imaging of a working catalyst by scanning transmission X-ray microscopy,” Nature 456, 222 (2008), doi:10.1038/nature07516.
ALS SCIENCE HIGHLIGHT #180