In the earth’s troposphere, which blankets the planet surface where we live and breathe, dust particles, gas molecules, water vapor, and sunlight provide the ingredients for a multitude of chemical reactions that can generate effects ranging from clouds and smog to acid rain and climate change. In 1949, chemist Rudolf Criegee proposed a novel chemical pathway for one such reaction, known as ozonolysis: the destruction of alkenes (a type of hydrocarbon), via reaction with ozone, a key pollutant in the troposphere. Although there has been much indirect evidence supporting Criegee’s mechanism, breakthrough research done at the ALS by chemists from Sandia National Laboratories, the University of Manchester, and Bristol University has for the first time directly measured reaction rates for so-called “Criegee intermediates,” elusive molecules formed at intermediate stages of ozonolysis. The surprising results may have important implications for subjects ranging from advanced engine design to air quality and climate modeling.

The research was conducted primarily at the Chemical Dynamics Beamline (Beamline 9.0.2) of the ALS, where vacuum ultraviolet light from the ALS synchrotron is used to investigate chemical reactions that occur during critical hydrocarbon oxidation processes (burning). Criegee intermediates—carbonyl oxides—are thought to play a key role in hydrocarbon autoignition chemistry, a field critical to advanced engine design. The detection of elusive Criegee intermediates is made possible by a unique apparatus designed by researchers from Sandia’s Combustion Research Facility. In 2008, at Beamline 9.0.2, the research team made the first direct observation of a Criegee intermediate. In the current work, the Sandia researchers report a new, more intense source of gas-phase Criegee intermediates.
The process begins with the laser photolysis of CH2I2 in a quartz reaction tube, generating the precursor reactant, CH2I. The CH2I reacts with a large excess of O2 to produce CH2OO—formaldehyde oxide—the simplest form of Criegee intermediate. The intense tunable light from the ALS then allows the researchers to use time-resolved photoionization mass spectrometry to discern the formation of different isomeric species—molecules that contain the same atoms but are arranged in different geometrical configurations. Thus the short-lived CH2OO produced in the reaction tube can be distinguished from its more stable isomers, such as formic acid (HCOOH), by their differing thresholds for photoionization.
The Manchester and Bristol researchers recognized that the apparatus could elucidate not only combustion reactions but also important tropospheric oxidation processes, such as ozonolysis. Ozonolysis, or the cleavage of carbon–carbon double bonds through reaction with ozone, plays a key role in the removal of unsaturated hydrocarbons (alkenes) from the troposphere. Rudolf Criegee proposed that ozonolysis of alkenes occurs via carbonyl oxide biradicals, now called Criegee intermediates in his honor. However, the indirectly derived, earlier reaction-rate coefficients—which govern how large a role these species will play in the atmosphere—were inconsistent and spanned many orders of magnitude.
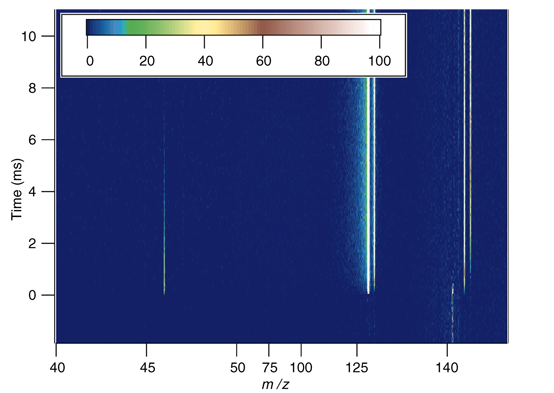
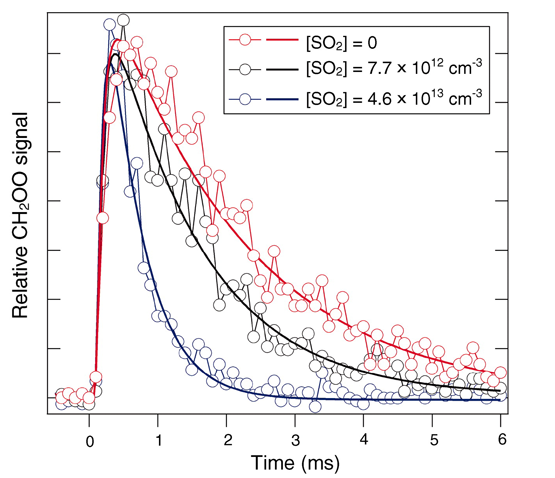
With the ability to generate and detect sufficient quantities of Criegee intermediates at Beamline 9.0.2, the research collaborators were able to determine the reaction-rate coefficients of reactions with key species such as water, SO2, NO, and NO2. In particular, the measurements show that the reactions of CH2OO with SO2 and NO2 are much more rapid than previously thought—50 to 10,000 times larger than estimates typically used in tropospheric models. Moreover, the Bristol and Manchester investigators demonstrated that these kinetic results imply a much greater role for carbonyl oxides in tropospheric sulfate and nitrate chemistry than models had assumed, a conclusion that will substantially impact existing atmospheric chemistry mechanisms. The ability to reliably produce Criegee intermediates will facilitate studies of their role in ignition and other oxidation processes as well as enable new detection methods beyond photoionization that might be deployed for in situ atmospheric sensing.
Contacts: David Osborn and Craig Taatjes
Research conducted by: O. Welz, J.D. Savee, D.L. Osborn, S.S. Vasu, and C.A. Taatjes (Sandia National Laboratories), C.J. Percival (University of Manchester, UK), and D.E. Shallcross (University of Bristol, UK).
Research funding: U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES), and the Natural Environment Research Council, UK. Operation of the ALS is supported by DOE BES.
Publication about this research: O. Welz, J.D. Savee, D.L. Osborn, S.S. Vasu, C.J. Percival, D.E. Shallcross, and C.A. Taatjes, “Direct Kinetic Measurements of Criegee Intermediate (CH2OO) Formed by Reaction of CH2I with O2,” Science 335, 204 (2012).
ALS SCIENCE HIGHLIGHT #243