The Nobel Prizes in scientific fields are awarded for discoveries or inventions that have “conferred the greatest benefit on mankind.” But often the full impact of a discovery takes decades to realize, during which the research is developed further and adopted by other scientists. Such was the case for the work of biochemist Paul Modrich, one of three recipients of the 2015 Nobel Prize in Chemistry “for mechanistic studies of DNA repair.” Berkeley Lab’s Advanced Light Source (ALS) was a core resource Modrich used to build on his earlier work.
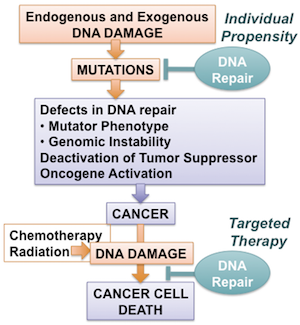
This year’s Nobel Prize has origins in the famous Nobel award from 53 years ago for solving the double-helix structure of DNA, which provides two complementary copies of genetic code and is essential to cell replication. However, James Watson and Francis Crick missed an important implication of their discovery. “They assumed DNA must be incredibly stable to maintain genome fidelity during replication,” says John Tainer, Berkeley Lab senior scientist and Professor of Molecular and Cellular Oncology at MD Anderson Cancer Center. “The trick is that you don’t maintain it. DNA is damaged constantly, but having two copies means if one strand becomes damaged, the complementary strand can be used as a template for repair.”
Understanding the mechanisms of that repair process has occupied the careers of many scientists since, including this year’s three Nobel laureates. Modrich’s contribution was in an area known as “mismatch repair,” which is coupled to replication. As cells divide, they frequently make mistakes or misspellings in the genetic code, incorrectly matching the base pairs of DNA’s two strands. Mismatch repair is the complex process that our bodies use to correct these misspellings. Learning how mismatch repair works—and how it can go wrong—is the key to stopping some types of cancer.
One effort seeking to better understand the mechanisms of mismatch repair has roots at Berkeley Lab and the ALS in particular. The Structural Cell Biology of DNA Repair Machines program (SBDR) involves more than 20 collaborators, including Modrich, from leading universities, national labs, and research institutes across the country. The list of collaborators reads like a who’s who of structural biology, biochemistry, and genetics—National Academy of Sciences members, fellows of every major professional society in the field, and now, a Nobel laureate. The program is funded by the National Cancer Institute of the National Institutes of Health and has been running for nearly 15 years, having been renewed twice.
Instead of duplicating work or creating competition among researchers, Tainer, the project leader, says the motivation behind the program was “to take the top researchers in DNA repair and link them together.” The group’s expertise, along with the technological capabilities available at Berkeley Lab, meant the program could tackle the grand challenge for cancer biology of achieving sufficiently detailed knowledge of DNA repair mechanisms to develop advanced cancer treatments with fewer adverse side effects. The knowledge Tainer’s team brought was in structural biology and mechanics, a new and crucial dimension for Modrich’s work.
Greg Hura, a beamline scientist at the ALS and member of the Molecular Biophysics and Integrated Bioimaging division at Berkeley Lab who has been with the project since its inception, describes Modrich’s earlier work on mismatch repair as a bit of trial and error. “He had some DNA synthesized with a mistake. Then he started adding proteins on top of it and assaying to see what was required for a fix. Eventually, he minimized the mixture to ten proteins essential to mismatch repair.” Modrich then knew the what, but not the how. “What are the roles of these guys? Who comes on first? Who identifies the mismatch? Why are there ten?” are the questions Tainer and Hura devote their time to answering.
An essential tool for answering those questions is ALS Beamline 12.3.1 (Structurally Integrated Biology for Life Sciences, or SIBYLS), which was built specifically for SBDR. SIBYLS is unique in the world, incorporating both small-angle x-ray scattering (SAXS) and crystallography capabilities at the same endstation. Tainer likens the information SIBYLS provides about biological structure to different contexts for looking at animals.
Crystallized structures, he says, are like the animals you would find in a natural history museum—frozen in a fixed position. Crystallography allows you to look in great detail at that frozen snapshot. X-ray scattering, on the other hand, is like looking at animals in the zoo. It allows examination of structures and their movement in an environment that is like their natural habitat. SIBYLS combines the two types of information to look at the ensemble of structural states under conditions that resemble those in cells.
According to Tainer, the collaboration and access to SIBYLS had a strong impact on Modrich’s research, resulting in more than 20 published papers. “He really got directly involved in the structural work with a paper on the x-ray scattering and some papers on crystallography. I think it changed his thinking about these systems.”
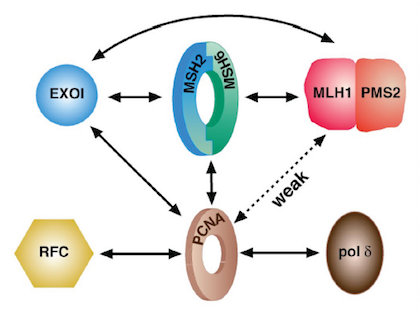
SIBYLS’s impact has reached far beyond Modrich and SBDR. Through additional funding from the U.S. Department of Energy and other sources, SIBYLS has studied hundreds of DNA repair processes. “Fifteen years ago, SAXS was a niche technique that only two or three labs were using,” says Hura. “Crystallography was really well developed, so it was kind of a risk to put SAXS on a crystallography beamline. This group really helped push SAXS to the point that we now have hundreds of labs using the technique.”
Both Tainer and Hura are quick to credit others as critical to SIBYLS’s existence and success. “[Modrich] really helped build this beamline,” explains Hura. “He’s central to why this collaboration got funded, and the work he was interested in doing helped drive our research.” Tainer says SIBYLS is “the design of a lot of very smart people at LBL. People like [ALS Deputy for Experimental Systems] Howard Padmore and all the machine physics guys and the engineers are the magic that meant we could do something special to address the biology of DNA repair. It’s a unique strength of LBL that this facility could be built and that we could involve such top-notch people to run a program for 15 years.”
Tainer and Hura hope SBDR will continue to be funded to probe the remaining mysteries of DNA repair, with the aim of unlocking more effective cancer treatments. “There are a lot of unanswered questions,” says Hura. “There’s still huge debate about how the broken spots are identified. We have pieces of the information, but we’re continuing to come up with rationally designed strategies to better understand and control the process.”