Burning of natural gas at oil and gas wells, called flaring, is a major waste of fossil fuels and a contributor to climate change. But to date, capturing the flared natural gas, estimated at some 140 billion cubic meters per year by the International Energy Agency, has not been economically feasible.
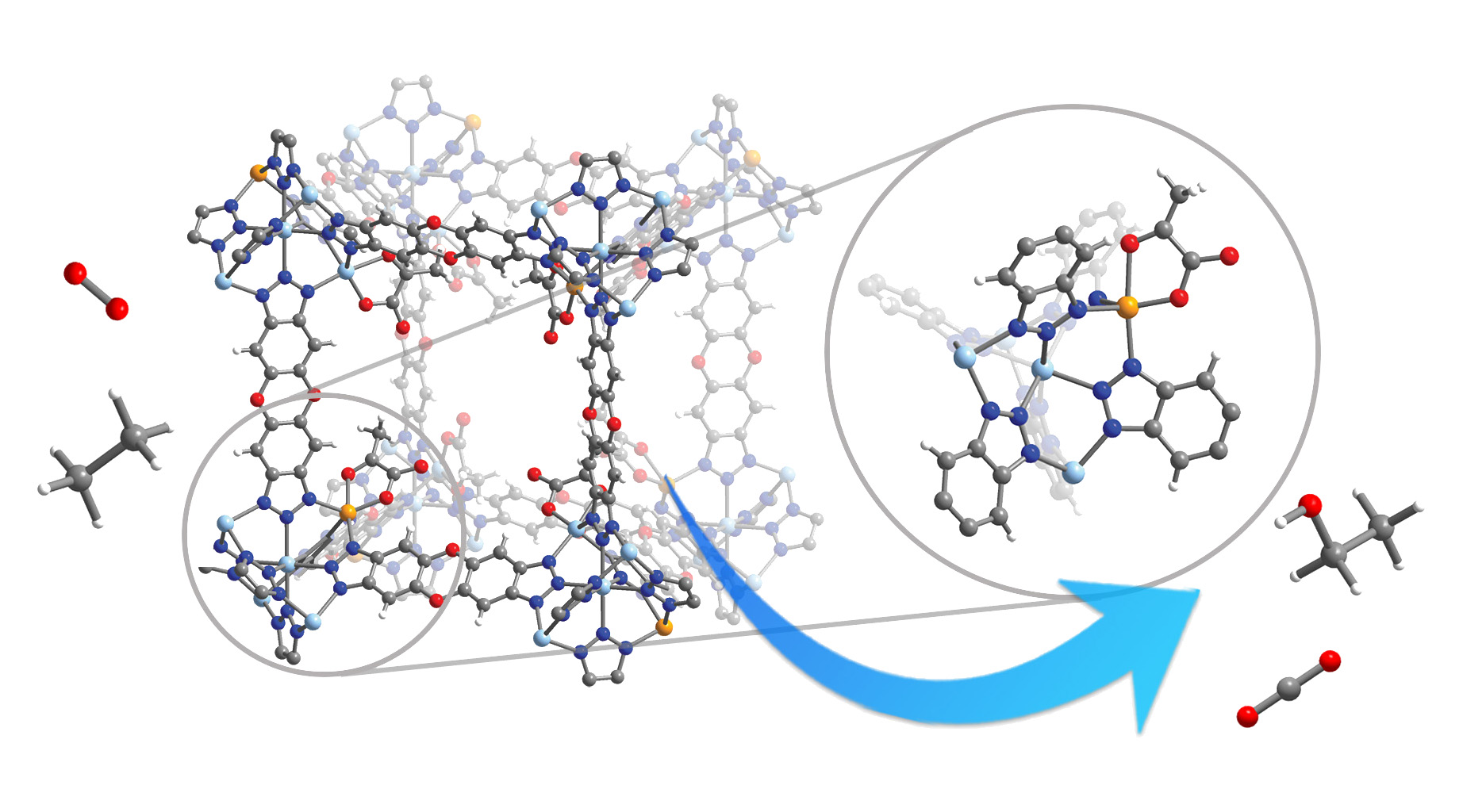
Researchers have now come up with a simple and green way to convert these gases—primarily methane and ethane—into economically valuable liquids, mostly alcohols like methanol and ethanol that can be used as feedstocks for numerous other petrochemical products. The new process mimics the way plants and animals produce energy by adding oxygen to carbon-hydrogen bonds, catalyzed by an enzyme centered around a reactive metal—in most cases, iron.
“We have a lot of natural gas wells here that are too small for building a large-scale facility around it to convert ethane to ethanol or methane to methanol,” said the study’s first author, Jonas Börgel, a UC Berkeley postdoctoral fellow. “This system is nice,” he said, “in that it is the first purely synthetic and not enzymatic process that can utilize oxygen at near ambient temperature to perform these reactions reminiscent of metalloenzyme reactivity.”
The synthetic process incorporates reactive iron sites into a metal-organic framework (MOF) that stabilizes the iron and allows easy entry of gas and easy exit of liquid alcohols. Spectroscopic analysis of the modified MOF confirmed that the iron(II) active site was acting just like the iron(II) site in TauD, a natural enzyme that converts hydrocarbons to alcohols. At the Advanced Light Source (ALS), single-crystal x-ray diffraction experiments at Beamlines 11.3.1 and 12.2.1 revealed details of the MOF’s pore structure and bonding details between key components.
While the process is still being perfected, Börgel said that if it proves efficient at producing alcohols with less energy input than current processes, it might also be useful in large-scale facilities, many of which produce megatons of alcohols per year from natural gas.
“We’re now trying to move from our Science paper, which was about a lot of fundamental chemistry, to working on making this a more amenable process,” Börgel said.
K. Hou, J. Börgel, H.Z.H. Jiang, D.J. Santalucia, H. Kwon, H. Zhuang, K. Chakarawet, R.C. Rohde, J.W. Taylor, C. Dun, M.V. Paley, A.B. Turkiewicz, J.G. Park, H. Mao, Z. Zhu, E.E. Alp, J. Zhao, M.Y. Hu, B. Lavina, S. Peredkov, X. Lv, J. Oktawiec, K.R. Meihaus, D.A. Pantazis, M. Vandone, V. Colombo, E. Bill, J.J. Urban, R.D. Britt, F. Grandjean, G.J. Long, S. Debeer, F. Neese, J.A. Reimer, and J.R. Long, “Reactive high-spin iron(IV)-oxo sites through dioxygen activation in a metal–organic framework,” Science 382, 547 (2023), doi:10.1126/science.add7417.
Adapted from the UC Berkeley press release, “Capturing wellhead gases for profit and a cleaner environment.”