Bimetallic cobalt-platinum (CoPt) nanoparticles are drawing attention in many areas of catalysis as scientists attempt to reduce precious metal content while maintaining optimum catalytic selectivity and reactivity. Cobalt, an important transition metal used for catalytic hydrogenation reactions of CO and CO2 to produce gaseous or liquid hydrocarbons, has a long history of use in the industrial process of producing synthetic fuels. Researchers explored the role of Pt in Co reducing and oxidizing, and found that the catalytic properties of monometallic and bimetallic nanoparticles of Co are closely related to the oxidation state of Co, which informs the prediction of cobalt oxidation states as reaction conditions are altered. This research also suggests the presence of a tetrahedral cobalt oxide that differs from the Co3O4 spinel structure.
CO oxidation is a model reaction that has been studied extensively; however, due to the technical difficulty of balancing ultra-high vacuum (UHV) conditions for soft x-ray with the chemical reaction conditions, few soft x-ray experiments have explored the chemical states of catalysts in different gas environments, especially up to the one bar pressure range. The oxidation state of a Co nanocatalyst is sensitive to the chemical environment and reaction temperature. By in situ mapping the oxidation states of Co under various conditions, this study contributes toward the understanding of the catalytic reactivity of Co nanoparticles.
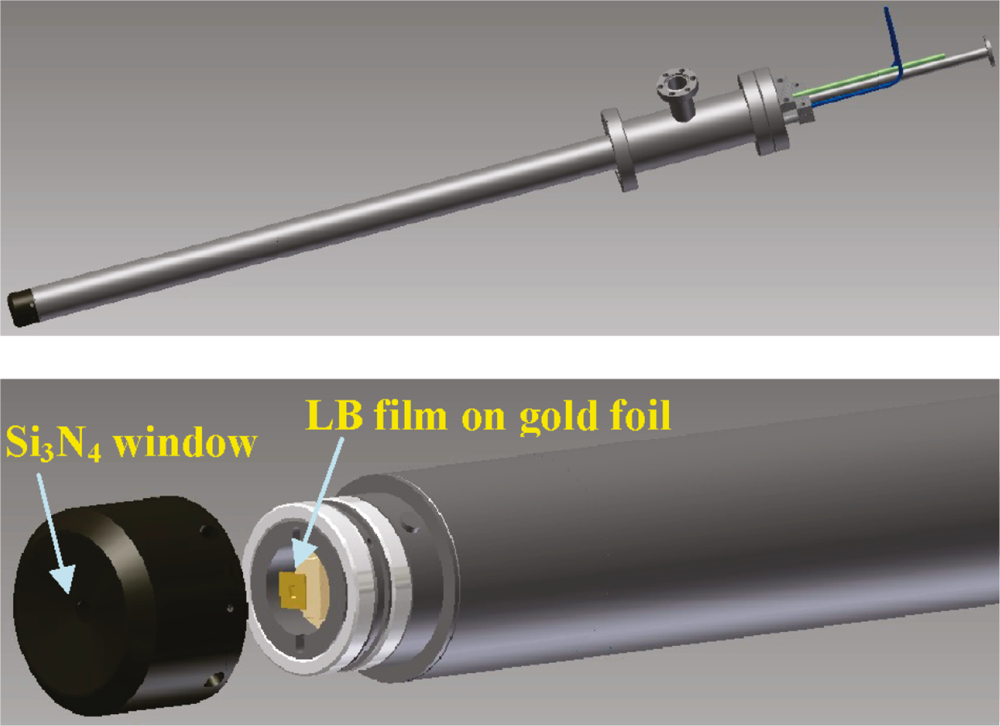
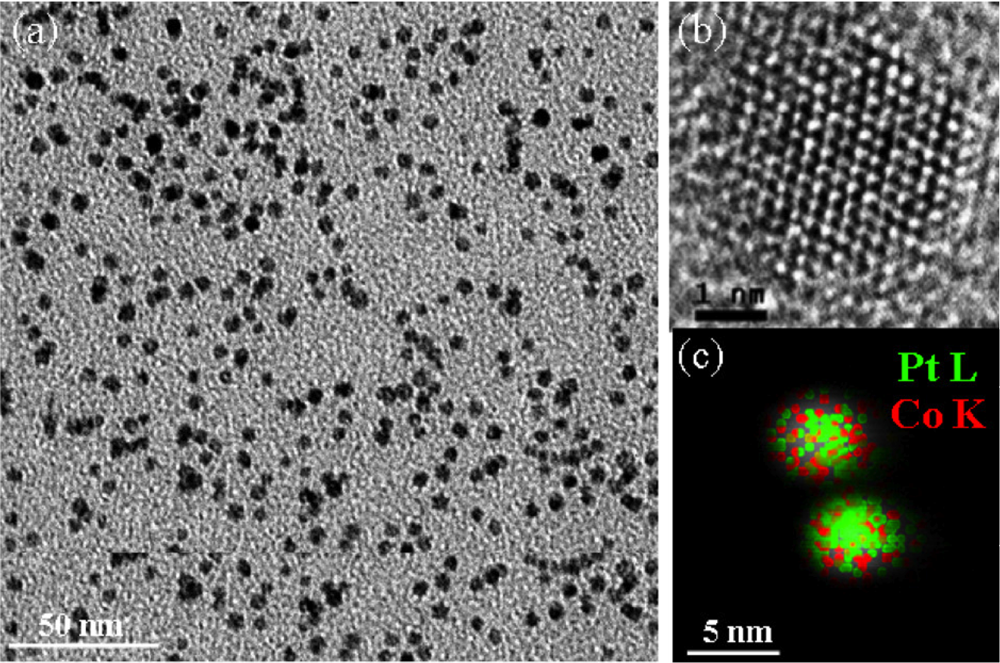
In situ near edge x-ray absorption fine structure spectroscopy (NEXAFS) was performed at ALS Beamline 7.0.1 to monitor the oxidation states of Co and CoPt nanoparticles 4 nm in size in the presence of H2 and O2 between 36 Torr and 1 bar, respectively. Synthesized nanoparticles were seen to be truly bimetallic, each comprising Pt and Co as demonstrated by STEM/EDS analysis. Fractions of Co oxidation states were mapped under the cycling total pressure.
Comparing the reducibility and oxidation states of Co in Co and CoPt nanoparticles, scientists found that Pt has a substantial impact on the oxidation state and structure of Co. Pt keeps Co in a more reduced state in the low O2 partial pressure range, and Co is much easier to reduce when alloying with Pt. When O2 partial pressure is increased above 4.8 Torr, Co0 and Co2+ fractions in the CO and CoPt nanoparticles tend to converge, while Co retains a much larger amount of the Co3+ fraction in the Co nanoparticles. As the pressure is decreased, Co3+ disappears and the fraction of Co2+ returns partially toward its original state. The incomplete reversal in the state of cobalt as the overall reaction pressure is lowered again could originate in a number of underlying reasons. The ratio of all gases was kept constant (O2:CO:He = 1.4:1:28).
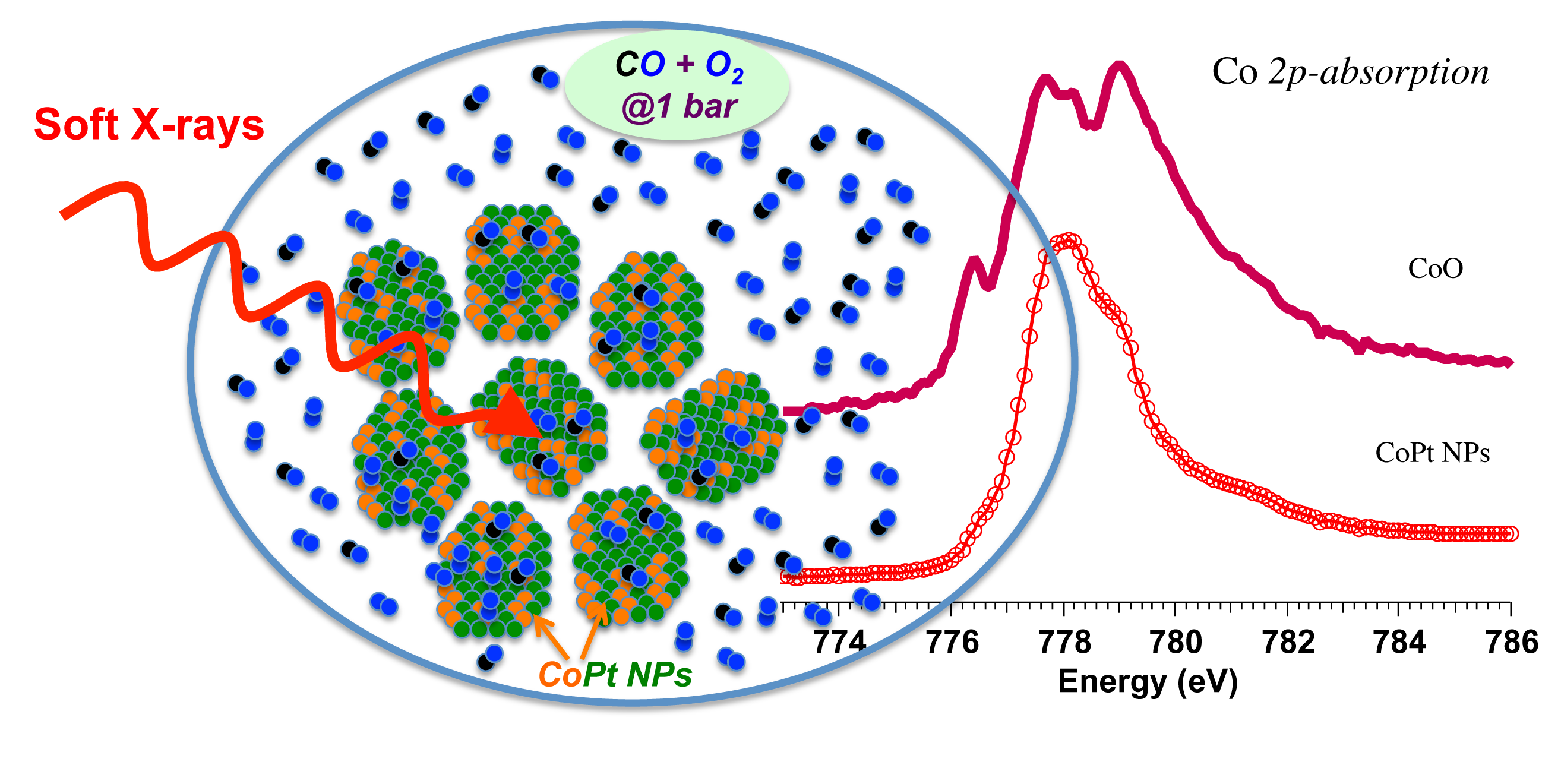
Prior to CO oxidation both nanoparticle samples were treated in 1 atm H2 at 125°C. Under reaction conditions a significant exchange of Co0 (initially 100% and 80% for CoPt and Co, respectively) to Co2+ states occurs as the reactant pressure is increased. Near the maximum pressure, some cobalt in the CoPt samples is fully oxidized to Co3+.
Comparing the reducibility and oxidation states of Co in Co and CoPt nanoparticles, scientists found that Pt has a substantial impact on the oxidation state and structure of Co. Pt keeps Co in a more reduced state in the low O2 partial pressure range, and Co is much easier to reduce when alloying with Pt. When O2 partial pressure is increased above 4.8 Torr, Co0 and Co2+ fractions in the CO and CoPt nanoparticles tend to converge, while Co retains a much larger amount of the Co3+ fraction in the Co nanoparticles. As the pressure is decreased, Co3+ disappears and the fraction of Co2+ returns partially toward its original state. The incomplete reversal in the state of cobalt as the overall reaction pressure is lowered again could originate in a number of underlying reasons. The ratio of all gases was kept constant (O2:CO:He = 1.4:1:28).
The 3d metal L edge soft x-ray absorption provides detailed features that can be resolved better compared to K edge absorption, making soft x-ray absorption a powerful tool when exploring the oxidation states of the catalysts since the fine structure is sensitive to the oxidation states.
The major result is that at high pressure of O2 and CO, cobalt’s oxidation state tends to be similar between pure Co and CoPt alloy nanoparticles. This is expected since it is in the O2 rich reaction condition. CO tends to block O2 adsorption less on the pre-oxidized Co than on the pre-reduced Co. Since more O2 is adsorbed when cobalt is already oxidized, Co in the CoPt alloy nanoparticles remains in a more oxidized state as pressure decreases than at the same partial pressure while the pressure is rising.
Contact: Jinghua Guo
Research conducted by: V. Iablokov (Universite Libre de Bruxelles and Berkeley Lab), N. Kruse (Universite Libre de Bruxelles), P.-A. Glans, J. Guo, and J.-L. Chen (Advanced Light Source) S. Alayoglu, S.K. Beaumont, Y. Li, C. Specht, V.V. Pushkarev, and F. Zheng (UC Berkeley), A.P. Alivisatos and G.A. Somorjai (Berkeley Lab and UC Berkeley).
Research funding: Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publications about this research: V. Iablokov, S.K. Beaumont, S. Alayoglu, V.V. Pushkarev, C. Specht, J. Guo, A.P. Alivisatos, N. Kruse, and G.A. Samorjai, “Size-Controlled Model Co Nanoparticle Catalysts for CO2 Hydorgenation: Synthesis, Characterization, and Catalytic Reactions,” Nano Letters 12, 3091 (2012); and F. Zheng, G.A. Somorjai, S. Alayoglu, J. Guo, V. Pushkarev, Y. Li, P.-A. Glans, and J.-L. Chen, “In-situ X-ray Absorption Study of Evolution of Oxidation States and Structure of Cobalt in Co and CoPt Bimetallic Nanoparticles (4nm) under Reducing (H2) and Oxidizing (O2) Environments,” Nano Letters 11, 847 (2011).
ALS SCIENCE HIGHLIGHT #264