Particles or aerosols can be directly released into the atmosphere (dust storms, volcanic eruptions, sea spray) or formed by chemical reactions in the atmosphere. Regardless of how they formed, they transform as they are transported by the wind, showing signs of age by increasing in size, chemical complexity, and oxidation, as well as becoming more mixed.
One way to monitor aerosol age is to collect fresh particles near the source and older particles downwind, then compare these microscopic particles using imaging analysis. Scientists from the Environmental Molecular Sciences Laboratory at Pacific Northwest National Laboratory and from Lawrence Berkeley National Laboratory did this recently with sea salt particles that had been collected by aircraft during the 2010 Carbonaceous Aerosol and Radiative Effects Study (CARES). Chemical imaging techniques (STXM and NEXAFS) at ALS Beamlines 5.3.2.2 and 11.0.2 showed that the sea salt particles from the ocean became coated by weak organic acids during transport. The organic acids reacted with the particles through a unique mechanism that had been overlooked in atmospheric chemistry. The reactions release volatile HCl into the atmosphere, but because the organic acids are very weak, only a miniscule amount of HCl is initially lost. As the particles age further, however, multiple hydration–dehydration cycles ultimately drive all the Cl from the sea salt into the atmosphere.
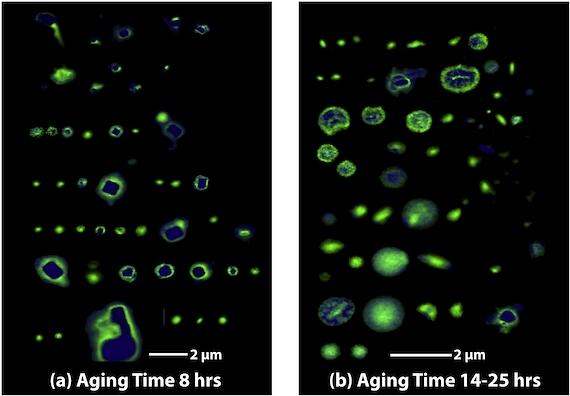
Work performed on ALS Beamlines 5.3.2.2 and 11.0.2.
Citation: A. Laskin, R.C. Moffet, M.K. Gilles, J.D. Fast, R.A. Zaveri, B. Wang, P. Nigge, and J. Shutthanandan, “Tropospheric chemistry of internally mixed sea salt and organic particles: Surprising reactivity of NaCl with weak organic acids,” J. Geophys. Res. 117, D15302 (2012), doi:10.1029/2012JD017743.