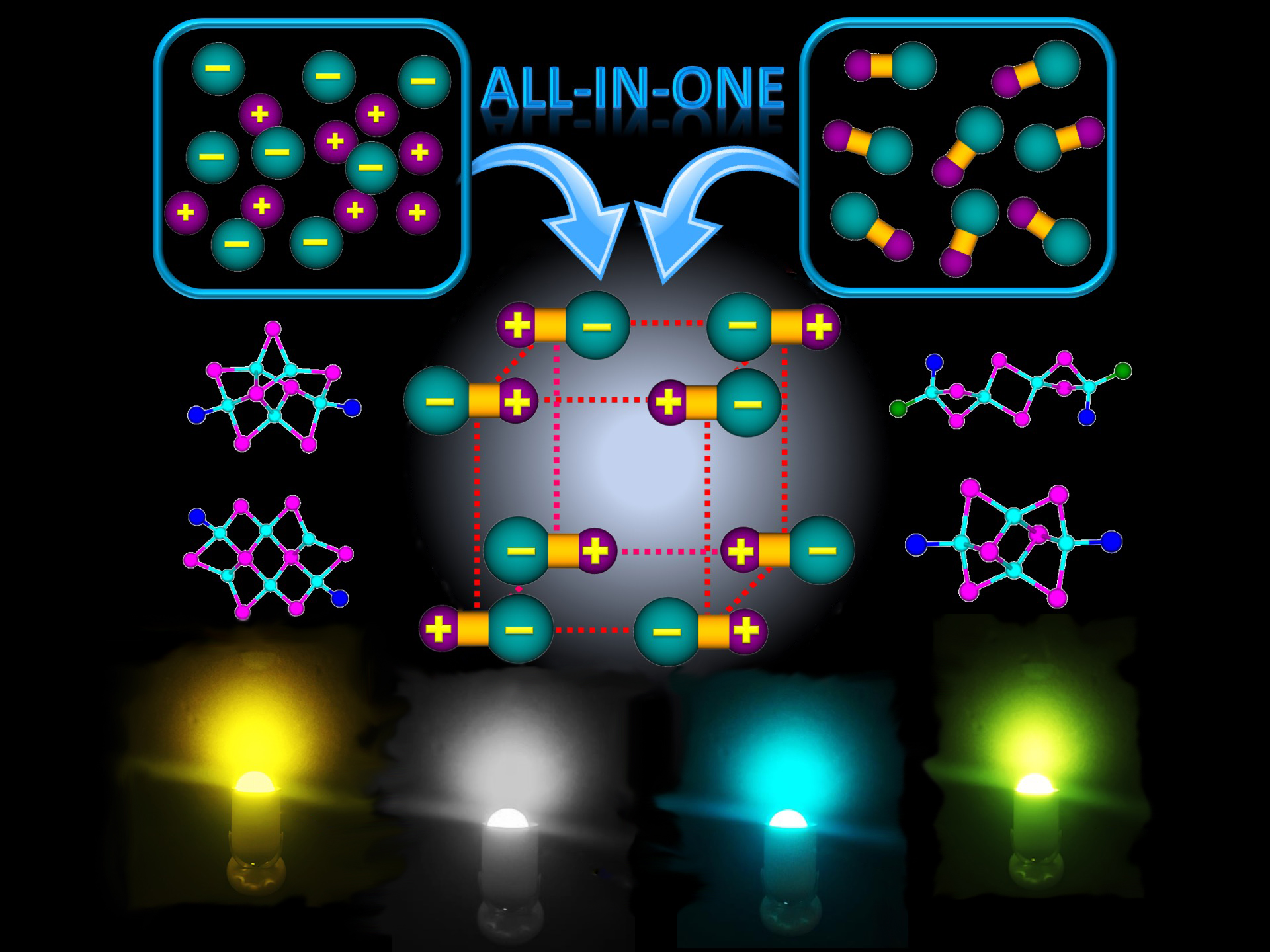
Conventional (incandescent and fluorescent) light bulbs are gradually being replaced by light-emitting diodes (LEDs) that last longer and are much more energy efficient. However, commercially available white LEDs currently rely on phosphor materials doped with rare-earth elements (REEs) that are increasingly costly and in short supply. A new class of hybrid phosphor materials based on copper (Cu) and iodine (I) shows promise as alternatives that are cost effective, highly abundant, and REE free.
The compounds are described as hybrid, or “all-in-one” (AIO), because they combine both ionic and covalent bonds within a single molecular cluster, providing greater structural stability than compounds with only covalent bonds and better emission properties than compounds with only ionic bonds. The compounds are composed of various negatively charged (CumIm+n)n- clusters and positively charged organic (nitrogen, phosphorus, or sulfur) ligands.
Small-molecule crystallography at ALS Beamline 11.3.1 was used to determine the single-crystal structure of all the compounds in this work. The results show that important and desirable properties (performance and durability) can be achieved by combining different structural components and chemical bonds in a single crystal.
With excellent performance and high stability comparable to benchmark commercial products, the AIO materials reported in this study could be good candidates for REE-free lighting phosphors in future commercial applications. More importantly, the discovery of AIO-type structures opens up new opportunities for designing many other hybrid materials with similar bonding characteristics.
Work performed at ALS Beamline 11.3.1.
W. Liu, K. Zhu, S.J. Teat, G.Dey, Z. Shen, L. Wang, D.M. O’Carroll, and J. Li, “All-in-One: Achieving Robust, Strongly Luminescent and Highly Dispersible Hybrid Materials by Combining Ionic and Coordinate Bonds in Molecular Crystals,” J. Am. Chem. Soc. 139, 9281 (2017), doi:10.1021/jacs.7b04550.