Microbiomes are communities of microbes that share our bodies, inside and out, and can be important to maintaining health and well-being. The web of relationships involved is incredibly complex—varying not just from species to species, but from individual to individual—making the job of untangling the interdependencies extremely challenging.
The microbiome of the fruit fly, Drosophila melanogaster, has been studied for over a century and is relatively simple in its composition compared to the mammalian gut microbiome. Nevertheless, how the fly-gut microbiome is regulated remains unclear.
In this work, researchers found that fruit flies have a specialized organ whose purpose is to select, maintain, and control bacteria that are used to benefit the fly. In order to make this discovery, they first had to catch wild Drosophila flies and isolate their gut bacteria. Next, they put fluorescent protein markers in the bacteria. They then used these engineered strains to image their localization in the gut.
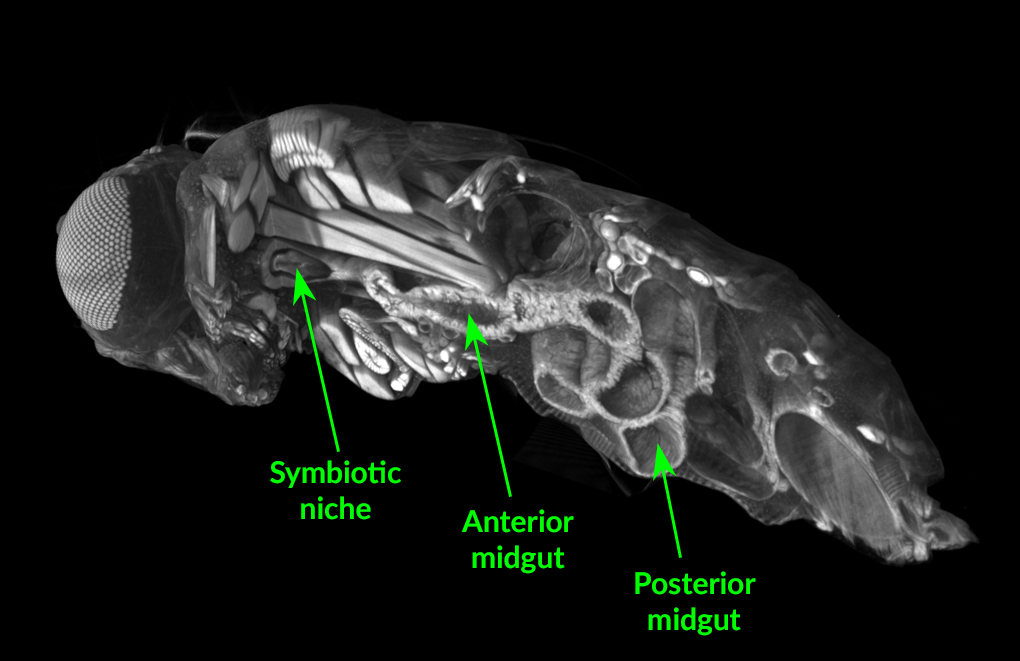
“We found that they all go to a specific physical space in the fly foregut, which we named the symbiotic niche,” said William Ludington, a microbial ecologist at the Carnegie Institution for Science. “There has been little study of the adult fly foregut in general and the symbiotic niche in particular. After over a hundred years of work, we never knew that flies had a specialized tissue to carry bacteria.”
The foregut is delicate, and the researchers wanted to understand its structure and how that changes in response to colonization by bacteria. For this, they used x-ray micro-computed tomography at ALS Beamline 8.3.2. They found that the symbiotic niche greatly expands in size when it is colonized by bacteria, indicating that it is responsive to symbionts.
It turns out that initial colonization by one particular bacterial species, Lactobacillus plantarum, physically remodels the niche, which favors secondary colonization by unrelated bacteria in the Acetobacter genus.
The results provide a mechanistic framework for understanding the establishment and stability of a multispecies intestinal microbiome and will help dissect the host mechanisms of host-microbe gut symbiosis.
R. Dodge, E.W. Jones, H. Zhu, B. Obadia, D.J. Martinez, C. Wang, A. Aranda-Díaz, K. Aumiller, Z. Liu, M. Voltolini, E.L. Brodie, K.C. Huang, J.M. Carlson, D.A. Sivak, A.C. Spradling, and W.B. Ludington, “A symbiotic physical niche in Drosophila melanogaster regulates stable association of a multi-species gut microbiota,” Nat. Commun. 14, 1557 (2023), doi:10.1038/s41467-023-36942-x.