The Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) has a long history of leadership in energy storage innovation, most recently with a game-changing Energy Storage Summit. Now, researchers have marked another advancement—a new methodology that helps to characterize processes at the interfaces between electrodes and electrolytes, with an eye toward bringing increased safety, lifetime, and energy density to the next-generation solid-state battery market.
A team in Berkeley Lab’s Energy Storage and Distributed Resources Division and the Advanced Light Source (ALS) used infrared nanospectroscopy with unparalleled spatial and chemical imaging capabilities to better understand and overcome persistent challenges that solid-state batteries face during operation.
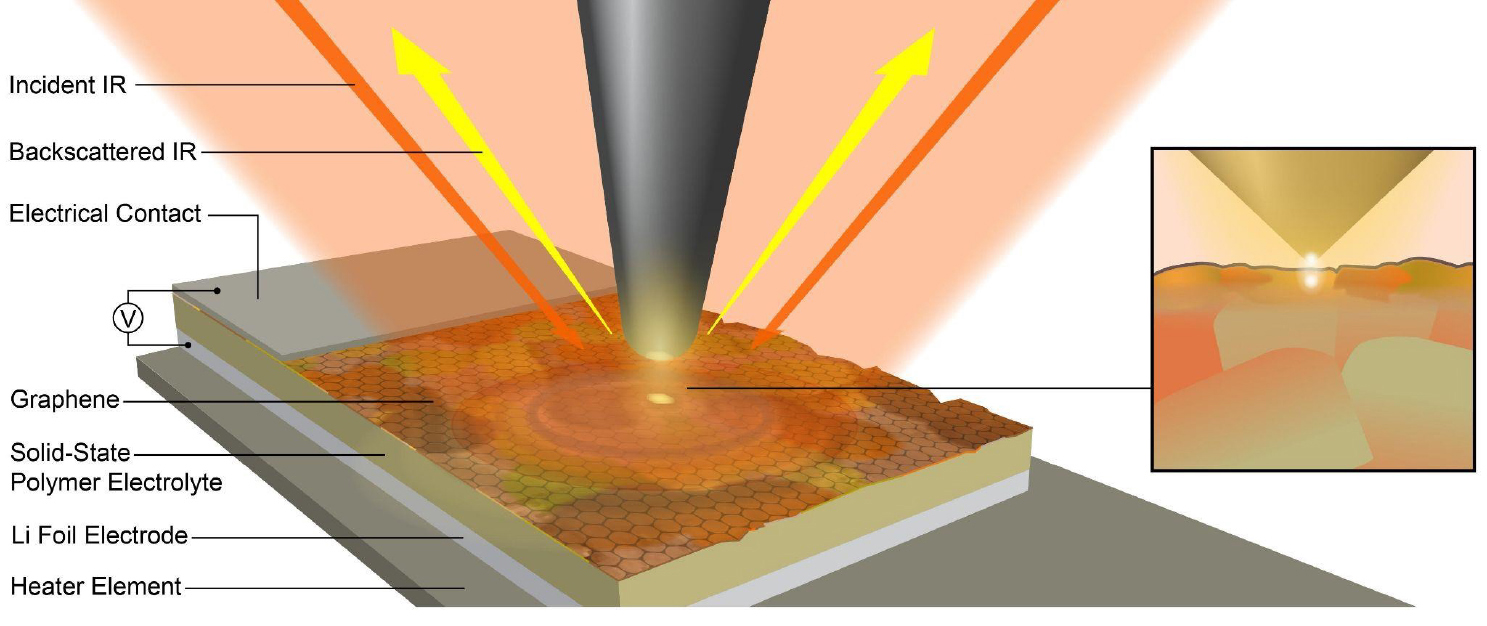
“Critically important to battery function and operation are the interfaces where two different materials adjoin, like the anode and electrolyte,” said Jonathan Larson, a postdoc on the research team and co-first and co-corresponding author of the published work. “During operation, a mysterious material thin film forms within interfaces (referred to as an “interphase”) which allows lithium ions to shuttle across the interface—enabling the battery to charge or discharge—but blocks electrons, which would cause the battery to rapidly degrade.”
In the past, researchers needed to take them apart to see what was going on. The team’s novel method, implemented at ALS Beamline 2.4, used an electrode made of a 1-atom-thin layer of carbon, allowing them to look through that layer and directly study interphase formation in its native environment with nanoscale spatial resolution and chemical selectivity.
“With this process, we can just look through the electrode side of the interphase between the electrode and electrolyte to evaluate what is going on inside,” Larson said. “This allows us unprecedented access to the structures. As this is used more frequently, we’ll have a better understanding of the solid electrolyte interphase, thereby feeding info back to engineering colleagues who can then engineer next-generation batteries.”
With the information they gather, the team hopes to bring a better understanding of what works well in conventional batteries to solid-state technologies.
X. He, J.M. Larson, H.A. Bechtel, and R. Kostecki, “In situ Infrared Nanospectroscopy of the Local Processes at the Li/Polymer Electrolyte Interface,” Nat. Commun. 13, 1398 (2022), doi:10.1038/s41467-022-29103-z.
Adapted from a news article by Berkeley Lab’s Energy Storage and Distributed Resources Division, “Looking Inside a Battery.”