SCIENTIFIC ACHIEVEMENT
Using the Advanced Light Source (ALS), researchers detected nanoscale deposits of elemental copper and iron in brain tissues isolated from Alzheimer’s disease subjects.
SIGNIFICANCE AND IMPACT
The discovery suggests new directions of study to determine the role that elemental metals might play in neurochemistry, neurobiology, and the development of neurodegenerative disease.
A closer look at the Alzheimer’s brain
Over a century ago, German psychiatrist and neuropathologist Alois Alzheimer examined the brain tissue of a woman who died exhibiting memory loss, language problems, and unpredictable behavior. One of the things he noted in his examination was a buildup of material—amyloid plaques—later understood to consist of naturally occurring proteins that accumulate between neurons and disrupt their signaling.
Now, an international team led by researchers from Keele University and Warwick University have extended the examination of Alzheimer’s-affected brain tissue to the nanoscale level, using x-ray spectromicroscopy at the ALS and Diamond Light Source. The results revealed the unexpected presence of elemental forms of copper and iron in amyloid plaques isolated from Alzheimer’s disease subjects, raising new questions about the role of metal biochemistry in the human brain.
Copper and iron brain chemistry
Copper and iron play a critical role in normal brain function. Numerous enzymes and proteins containing positively charged (oxidized) copper and iron (Cu+, Cu2+, Fe2+, and Fe3+) control key metabolic and neurological processes. However, metallic (unoxidized) copper (Cu0) and iron (Fe0) have chemical and magnetic properties that are distinctly different from those of their oxidized counterparts. Moreover, the metallic surfaces are highly reactive and will easily combine with oxygen to form the positively charged oxides.
Disruptions in the balance (homeostasis) between metals in these chemical states have been implicated in the development of multiple neurodegenerative disorders, including Alzheimer’s disease and the formation of amyloid plaques. A deeper understanding of metal homeostasis in the brain, and, in particular, the role of specific oxidation states, requires chemically sensitive measurements at nanoscale resolution.
High-resolution chemical specificity
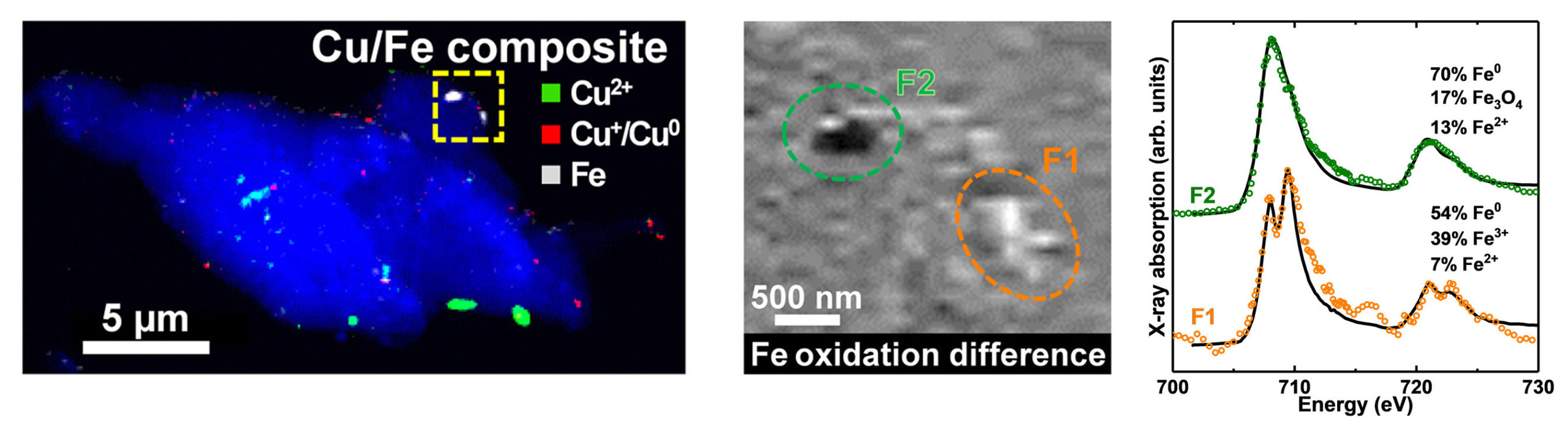
At both the ALS and Diamond, scanning transmission x-ray microscopy (STXM) experiments were performed on amyloid plaque cores isolated from the brain tissues of deceased Alzheimer’s patients. The chemical sensitivity and nanoscale spatial resolution offered by ALS Beamline 11.0.2.2 allowed the researchers to determine the chemical composition of extremely small samples in a way that revealed both the precise distribution of specific elements and the extent to which they were oxidized.
The results revealed the presence of nanoparticles with high concentrations of metallic iron and copper, along with different chemically reduced states, within the same amyloid plaque. This suggests that aggressive oxidation-reduction (redox) reactions may be occurring within these structures. In addition, magnetic dichroism measurements revealed iron deposits with strong magnetic polarization, a finding that could affect certain forms of magnetic resonance imaging (MRI) and help with diagnosis and monitoring of disease progression.
The researchers do not rule out the possibility that these elemental nanoparticles may exist outside of the amyloid plaques, possibly even in the brains of healthy individuals. Thus, the next steps will be to determine if the metallic particles are only (or at least predominantly) found in areas of disease pathology such as Alzheimer’s disease plaques. In general, this line of research will help scientists to better understand the complex biochemistry of the brain and, in particular, the role that amyloid plaques and transition metals—both current targets for treatment—play in Alzheimer’s disease.
Contact: James Everett
Researchers: J. Everett (Keele University and University of Warwick, UK); F. Lermyte (University of Warwick, UK, and Technical University of Darmstadt, Germany); J. Brooks, V. Tjendana-Tjhin, I. Hands-Portman, J.M. Donnelly, P.J. Sadler, P.B. O’Connor, and J.F. Collingwood (University of Warwick, UK); G. Plascencia-Villa and G. Perry (University of Texas at San Antonio); K. Billimoria (University of Warwick and LGC Ltd., UK); X. Zhu (Case Western Reserve University); and N.D. Telling (Keele University, UK).
Funding: Engineering and Physical Sciences Research Council (UK), University of Warwick alumni donations, Fulbright-Warwick Scholarship, Kleberg Foundation, National Institutes of Health, Alzheimer’s Association, The Lowe Foundation, and Semmes Foundation. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: J. Everett, F. Lermyte, J. Brooks, V. Tjendana-Tjhin, G. Plascencia-Villa, I. Hands-Portman, J.M. Donnelly, K. Billimoria, G. Perry, X. Zhu, P.J. Sadler, P.B. O’Connor, J.F. Collingwood, and N.D. Telling, “Biogenic metallic elements in the human brain?” Sci. Adv. 7, eabf6707 (2021), doi:10.1126/sciadv.abf6707.
ALS SCIENCE HIGHLIGHT #448