Electronic cigarettes emerged in the early 2000s as basic “cig-a-likes” that mimicked the shape of traditional cigarettes. They have since evolved into complex, battery-powered electronic devices with e-liquid chemistries fine-tuned to affect how nicotine is delivered, dosed, and experienced.
Recent e-cigarette additives like benzoic acid can change the acid-base chemistry of nicotine in the inhaled aerosol by adding a proton. The result is a smoother pull with less throat irritation, allowing users to more comfortably inhale higher doses of nicotine. In encouraging higher dosing, these additive-laced vaping products are viewed as fueling an epidemic of youth vaping.
“Understanding the aerosolized chemical species of nicotine in the presence of additives like benzoic acid is particularly important for determining its effects once it reaches the body’s respiratory tissues,” said the study’s principal investigator, Hugo Destaillats, a senior scientist in Berkeley Lab’s Energy Analysis and Environmental Impacts Division. “Our study describes how much of the delivered nicotine is present in the protonated form in the aerosol particles, and how this speciation differs between the core and surface of particles.”
To gain such a rare, detailed glimpse into e-cigarette chemistry and determine how much protonated nicotine is delivered by vaping, researchers used x-ray photoelectron spectroscopy (XPS) and near-edge x-ray absorption fine structure (NEXAFS) spectroscopy at Advanced Light Source (ALS) Beamine 9.0.1.
One of the most striking findings was that nicotine’s acid-base equilibria in water-based aerosols is very different from its well-known behavior in aqueous solution in the same pH range. Furthermore, the researchers found important differences in the degree of protonation of nicotine present at the surface, with respect to that in the core of the particles.
Because acid-base chemistry affects nicotine uptake and users’ perceptions, results from this study can help scientists to better understand how nicotine is delivered to the tissues and fluids in the respiratory system. For example, these results can help formulate aerosols delivered to model systems in future in vitro and in vivo studies addressing nicotine deposition and uptake. Describing the role of acidifying additives is needed to support public health policy interventions.
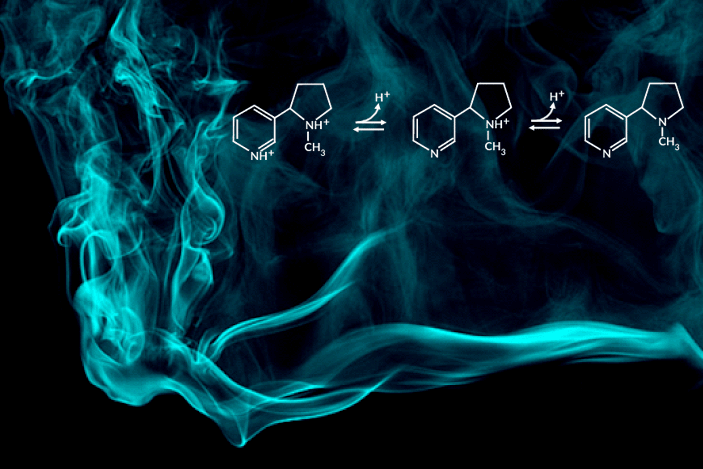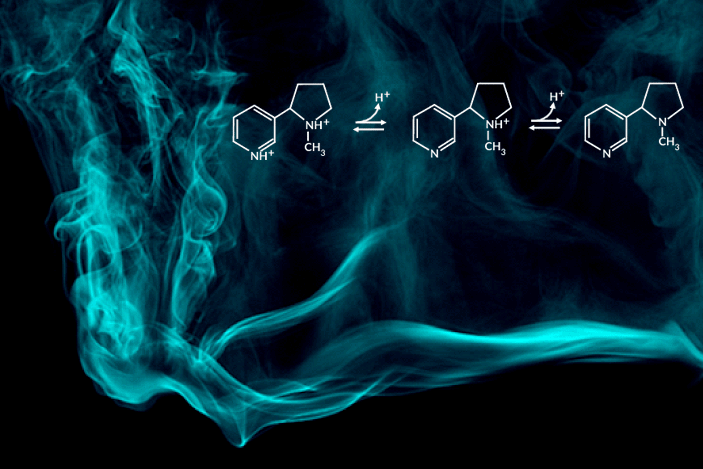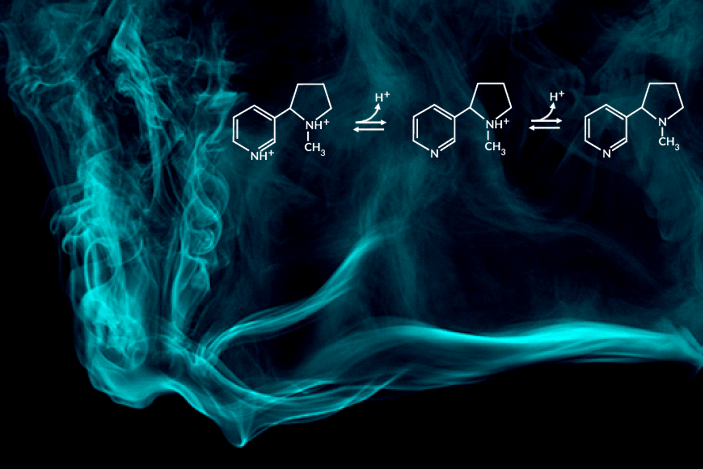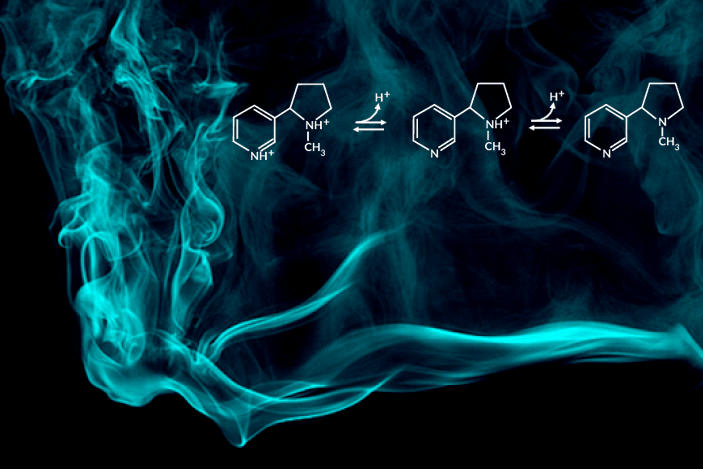
C. Weeraratna, X. Tang, O. Kostko, V.H. Rapp, L.A. Gundel, H. Destaillats, and M. Ahmed, “Fraction of free-base nicotine in simulated vaping aerosol particles determined by X-ray spectroscopies,” J. Phys. Chem. Lett. 14, 1279 (2023), doi:10.1021/acs.jpclett.2c03748.
Adapted from Berkeley Lab’s Energy Technologies Area highlight, “Berkeley Lab Researchers Shine a Light on Vaping Chemistry.”