SCIENTIFIC ACHIEVEMENT
At the Advanced Light Source (ALS), researchers found that when an ion-conducting polymer composite is placed in an electric field, it forms ion-rich hotspots that continue to grow for hours after the field is removed.
SIGNIFICANCE AND IMPACT
The study opens a new path to understanding the dynamic structure of composite materials for smaller, lighter batteries.
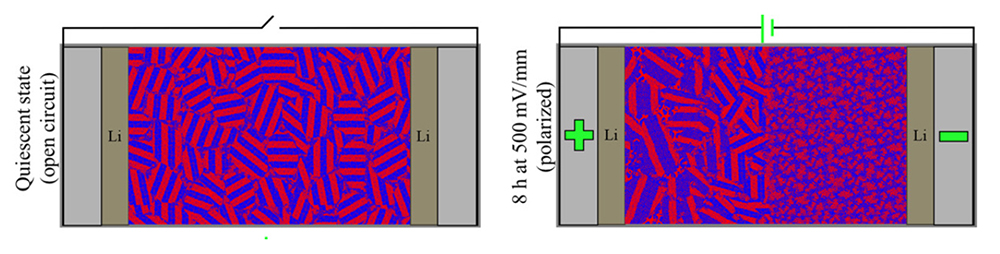
Battery bottlenecks
To develop lighter, longer-lasting batteries for mobile devices like cell phones and cars, scientists are on the hunt for new electrolyte materials. Electrolytes separate the positive and negative electrodes of a battery while allowing ions to travel back and forth. Lithium—a light, energy-dense metal, is a theoretically ideal material for electrodes. Unfortunately, electrolytes paired with lithium develop branch-like lithium growths (dendrites) that allow contact between the electrodes, short-circuiting the cell.
One way to avoid this is to develop electrolytes with nanostructures that disrupt dendrite growth. However, direct observation of the ion-transport mechanics of such systems is difficult, resulting in a bottleneck to further progress. In this work, researchers used x-ray scattering to measure structural changes in a model electrolyte under an applied electric field. The ability to spatially resolve the electrolyte’s nanostructure under realistic conditions will help produce insights needed for future breakthroughs.
A morphing block copolymer
The model electrolyte was a block copolymer—a plastic consisting of two types of polymers that self-organize into nanosized domains (blocks). One block (a glassy polystyrene) provides the mechanical rigidity needed to prevent the formation of dendrites, while the other (a gelatinous polyethylene oxide) provides the medium for ion conduction.
Normally, the two domains form a lamellar (layered) phase. By adding a source of dissolved lithium ions (a lithium “salt”) to the system, the researchers were able to correlate changes in the electrolyte’s nanostructure to salt concentration. At sufficiently high concentrations, the structure shifts from the lamellar phase to a “gyroid” phase in which the rigid polystyrene forms a sponge-like matrix with the gel-like polyethylene oxide filling the pores.
In situ polarization studies
To study how the electrolyte’s nanostructure changes under operating conditions, the researchers designed a special cell enabling small-angle x-ray scattering (SAXS) measurements of the electrolyte under an applied current at ALS Beamline 7.3.3. Unlike previous experiments that passed x-rays perpendicularly through the electrodes, this cell allowed the x-ray beam to scan between the electrodes, probing the electrolyte structure as a function of position as well as time. Analysis of the SAXS data—peak position, intensity, and shape—provided information about how the rigid block copolymer nanostructure changed when the current was on and for several hours after the current was turned off.
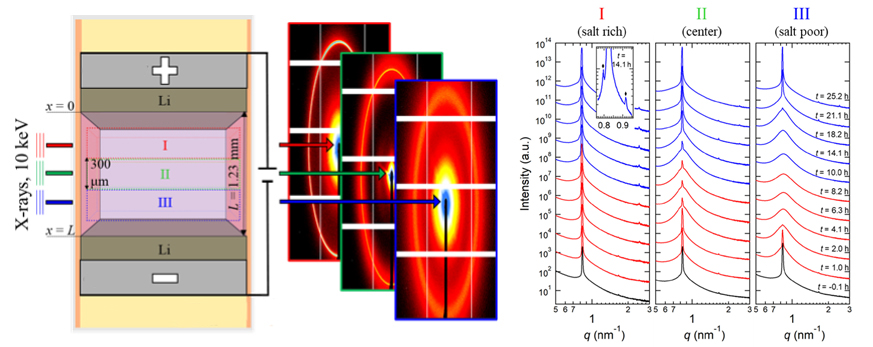
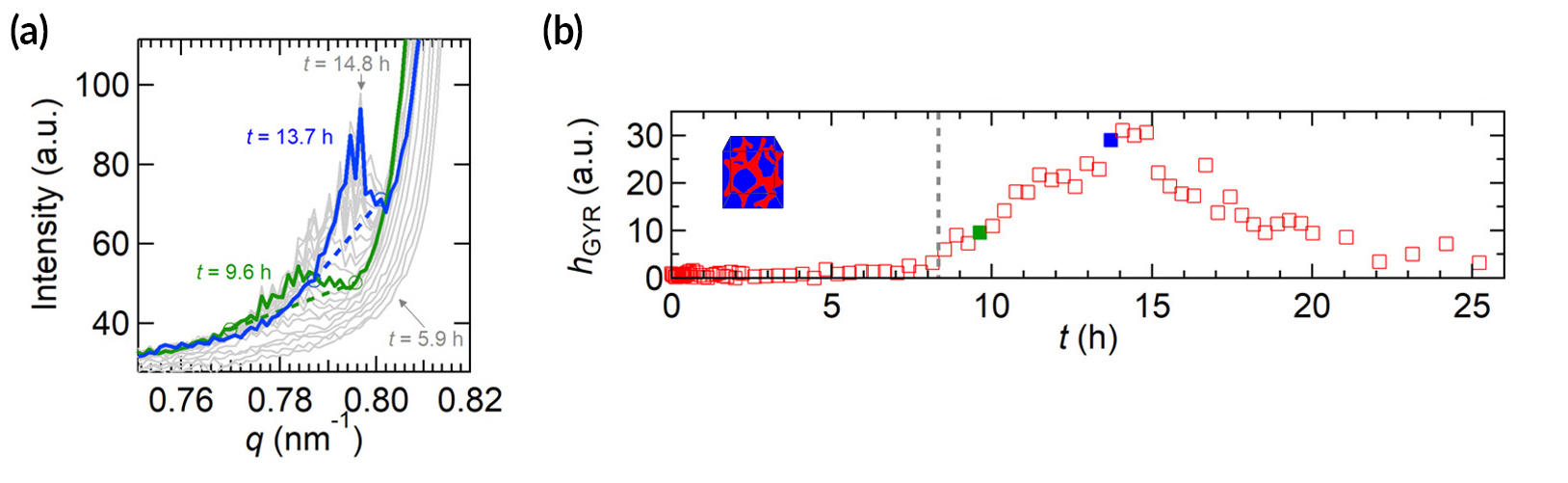
Ions hit a wall
The data revealed that, in an external electric field, the composite electrolyte developed a disordered phase near the negative electrode and a mix of lamellae and tiny pockets of gyroid phase near the positive electrode. The gyroid phase reflected the formation of undesirable “concentration hotspots” where salt ions, propelled by the current, accumulated after bumping into a polystyrene wall. Even more surprisingly, the gyroid phase continued expanding for six hours after the field was removed, a consequence of the buildup of extremely high salt concentrations in localized pockets that took time to dissipate.
Because all batteries—including those currently in use—include composite materials, results such as these help explain why some batteries fail unexpectedly. More generally, the methods described here can be used to explore the dynamic structure of other composite materials, with the long-term goal of understanding how to avoid problems such as concentration hotspots in practical systems.
Contacts: Nitash Balsara and Michael Galluzzo
Researchers: M.D. Galluzzo and N.P. Balsara (UC Berkeley and Berkeley Lab), W.S. Loo (UC Berkeley), and E. Schaible and C. Zhu (ALS).
Funding: U.S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office. Operation of the ALS is supported by DOE, Office of Science, Basic Energy Sciences program.
Publication: M.D. Galluzzo, W.S. Loo, E. Schaible, C. Zhu, and N.P. Balsara, “Dynamic Structure and Phase Behavior of a Block Copolymer Electrolyte under dc Polarization,” ACS Appl. Mater. Interfaces 12, 57421 (2020), doi:10.1021/acsami.0c16209.
ALS SCIENCE HIGHLIGHT #438