Our DNA encodes just 20 amino acids, enough to link into tens of thousands of proteins. Variations in their structure and behavior drive variability in nature, but many of their chemical properties have been difficult to study at the most fundamental level. Arginine, an amino acid that has more than 100 isomers, is fragile and becomes fragmented in the gas phase. Advancements in both technique and theory have now enabled researchers to determine the ionization energy of arginine, fundamental information that will inform chemical sensing technology for human health or environmental remediation.
To determine the ionization energy, researchers combined a technique developed at the Advanced Light Source (ALS) with new computational methods that model how water molecules affect arginine in solution. Vacuum ultraviolet (VUV) mass spectrometry and photoelectron spectroscopy at Beamline 9.0.2 provided insights into gas-phase arginine vaporized from aerosols and under various pH conditions. A combination of experiments and theory allowed the researchers to determine the ionization energy of gas-phase arginine and follow the effects of solvation (addition of water), such as stabilizing the molecular ion from fragmentation.
Expanding the suite of techniques and theories has deepened our fundamental understanding of biomolecules; knowing the ionization energy of arginine has implications for oxidation, reduction, and hydrogen bond formation in peptides and proteins. This knowledge can be extended to other soft x-ray studies relevant to crystallography and protein dynamics. In addition, these studies inform the development of molecular electronics and sensors that monitor processes relevant to health and environmental matters.
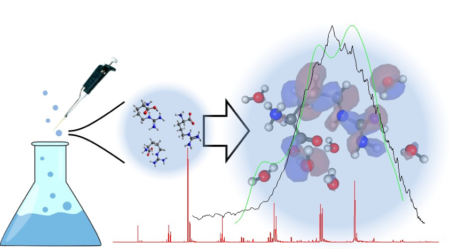
A. Barrozo, B. Xu, A. O. Gunina, M. I. Jacobs, K. Wilson, O. Kostko, M. Ahmed, and Ai. I. Krylov, “To Be or Not To Be a Molecular ion: The Role of the Solvent in Photoionization of Arginine,” J. Phys. Chem. Lett. 10, 1860–1865 (2019), doi:10.1021/acs.jpclett.9b00494.