Metallic lithium has properties that make it one of the best materials to use as an anode in rechargeable batteries. However, its high reactivity hinders broad commercialization of next-generation lithium-based batteries, including lithium-sulfur and lithium-air batteries. CO2 gas is part of the environment in which pristine lithium, intended for battery use, is stored prior to assembly. A better understanding of the interactions between lithium and surrounding gases will help in the design of strategies to stabilize lithium and move from lithium-ion technology to high-energy-density technologies based on lithium metal.
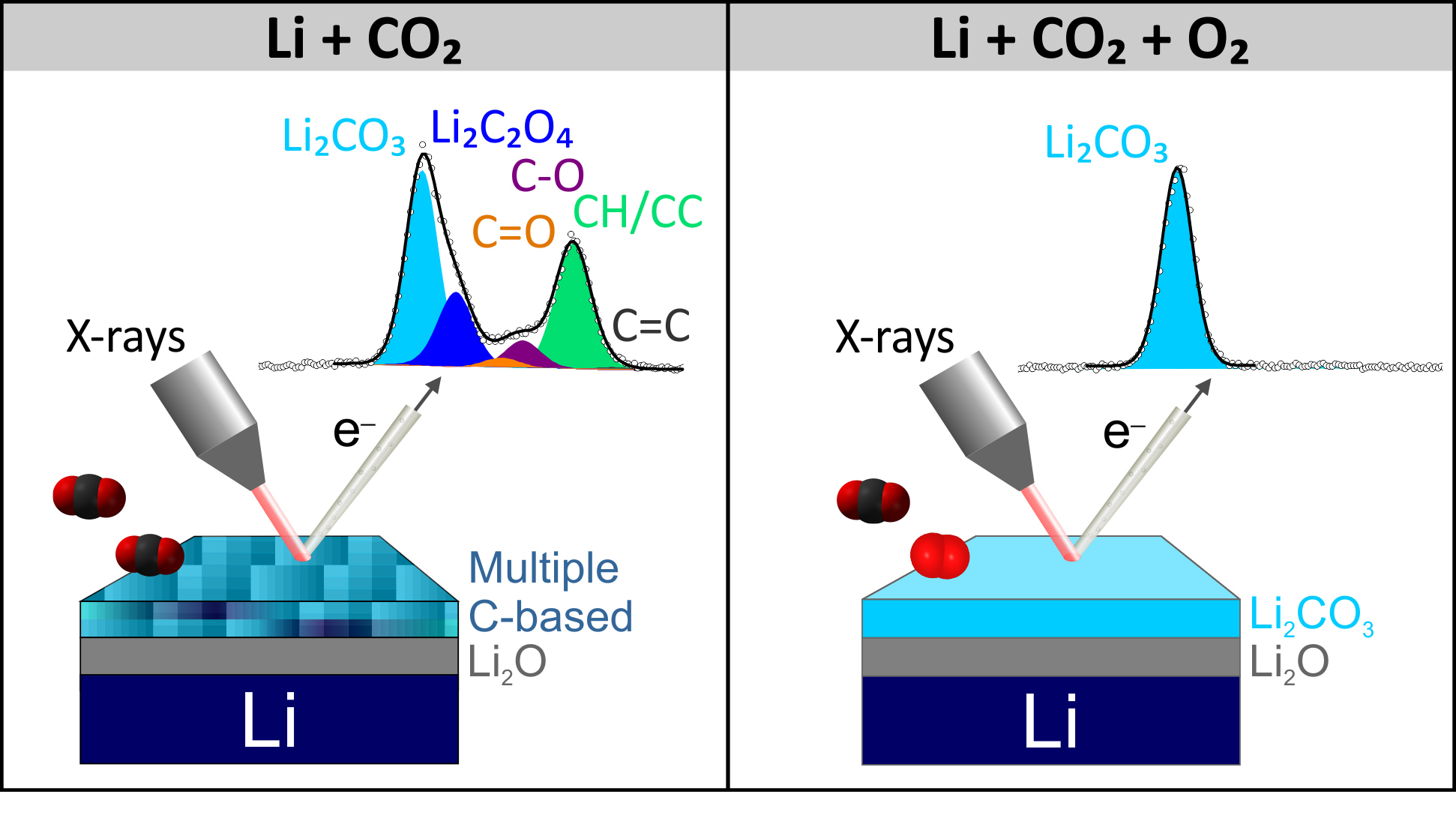
In this work, researchers studied how CO2 gas modifies the chemical composition of the lithium surface. Led by ALS doctoral fellow, Ane Etxebarria (now a postdoctoral fellow at the Fritz Haber Institute of the Max Planck Society), the researchers studied the surface reactivity of lithium with ambient-pressure x-ray photoelectron spectroscopy (APXPS) at Advanced Light Source (ALS) Beamline 9.3.2. This technique allowed them to follow surface reactions in situ, clarify the reaction pathway, and identify the main intermediates involved.
Interestingly, adding O2 gas to the CO2 environment promoted carbonate formation, bypassing the reaction pathway and avoiding the formation of intermediates. For electrode applications, reactions that result in a single product on the lithium surface are likely preferred to those leading to a mixture of products, because it avoids dendrite growth driven by differences in the ionic conductivity of surface species. More fundamental studies focused on the reaction of lithium metal with surrounding gases are essential if the high reactivity of the lithium surface is to be successfully controlled.
A. Etxebarria, D.-J. Yun, M. Blum, Y. Ye, M. Sun, K.-J. Lee, H. Su, M.Á. Muñoz-Márquez, P.N. Ross, and E.J. Crumlin, “Revealing in situ Li metal anode surface evolution upon exposure to CO2 using Ambient Pressure X-ray Photoelectron Spectroscopy,” ACS Appl. Mater. Interfaces 12, 26607 (2020); doi:10.1021/acsami.0c04282.