SCIENTIFIC ACHIEVEMENT
An atomically precise surface probe at the Advanced Light Source (ALS) helped researchers discover that a catalyst can be activated by tuning the composition of just one atomic surface layer.
SIGNIFICANCE AND IMPACT
The work sharpens our understanding of how surface changes can improve the production of hydrogen fuel from water using efficient catalysts made of inexpensive materials.
Splitting water for hydrogen fuel
Hydrogen is a clean-burning fuel that generates only water as a by-product, making it an attractive option for sustainable and renewable energy. To produce this fuel, hydrogen atoms in water must be separated from oxygen atoms, a process that can be driven electrochemically with the help of catalysts, which are used to speed up or otherwise improve the efficiency of chemical reactions.
Scientists are working to improve the economics of hydrogen as a fuel by developing more efficient catalysts composed of earth-abundant materials. Recent experiments have now revealed that one such catalyst can be activated by an unexpected transformation in a single atomic layer at its surface. Led by scientists from Stanford University and SLAC National Accelerator Laboratory, the research team applied a unique x-ray technique that was pioneered at the ALS, combined with related analyses, to home in on changes at the surface of the material.
A model perovskite catalyst
The catalyst investigated in this study, lanthanum nickel oxide (LaNiO3, or LNO), belongs to a class of materials known as perovskites, named after a natural mineral with a similar atomic structure. Perovskite-type oxides have the general chemical formula ABO3, where the selection of A and B provide “tuning knobs” for chemical and electrical properties. In addition, LNO is a model perovskite oxide with well-controlled bulk properties and an “experimental lever”—temperature—that enables researchers to modify the surface composition of LNO thin films by growing them at different temperatures (in this case, at 450 and 650 °C).
Standing-wave x-ray probe
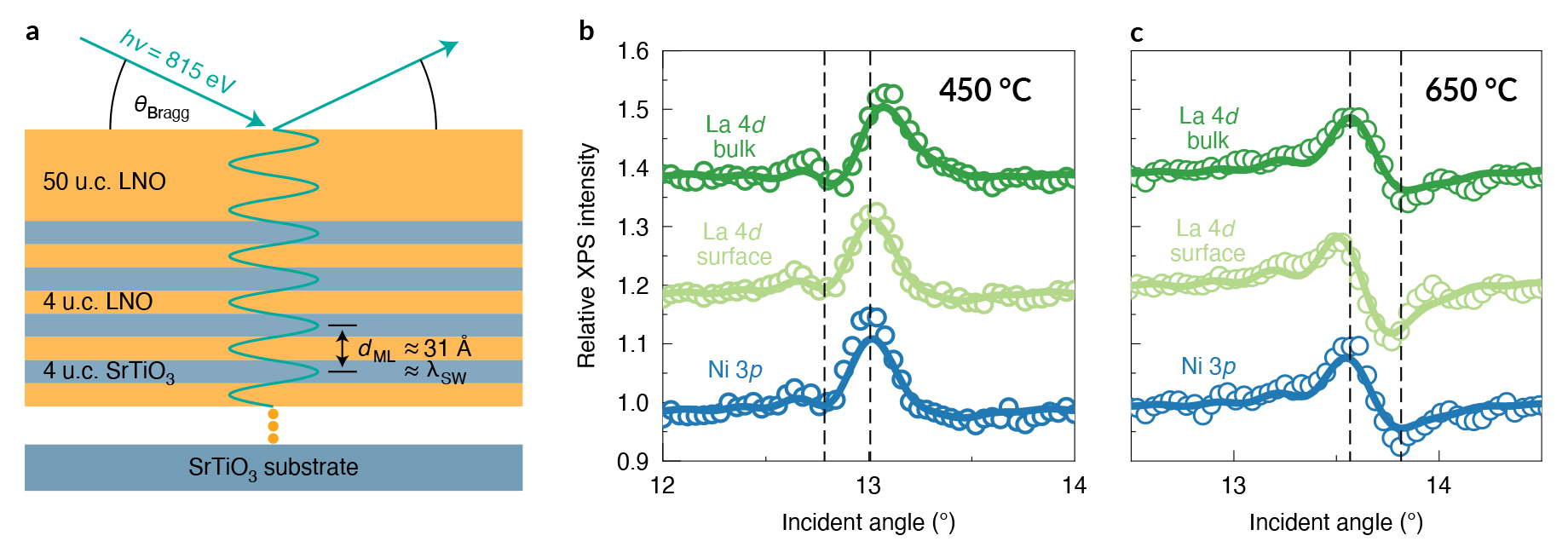
To study how the temperature difference affected the surface composition of the films, the researchers turned to standing-wave ambient-pressure x-ray photoelectron spectroscopy (SW-XPS or SWAPPS) at ALS Beamline 11.0.2. SW-XPS can profile the chemical environment of a surface with a depth resolution of 1–2 Å, an extremely fine level of precision on the interatomic scale.
The vertical position of the standing-wave antinodes—where photoelectrons are generated with the highest intensity—can be shifted by varying the x-ray incident angle (or by rocking the sample), producing depth-selective photoemission intensity modulations. Comparisons of the “rocking curve” spectra for various chemical species indicated that the LNO samples grown at different temperatures vary in exactly one topmost atomic layer—the surface is Ni-rich when prepared at 450 °C and La-rich when grown at 650 °C.
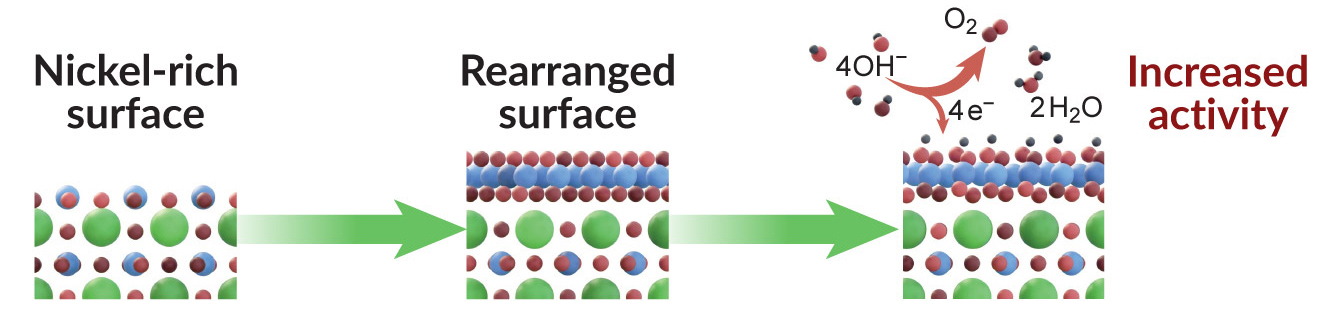
Catalytic pathway unlocked
Electrochemical measurements showed that the Ni-rich samples activated the water-splitting reaction at a lower energy than expected, and the reaction proceeded twice as fast compared to the La-rich surfaces, which did not transform into an active surface phase, even after tens of hours of operation.
Computer simulations performed at Berkeley Lab’s National Energy Research Scientific Computing Center (NERSC) indicated that the observed results were consistent with a structural transformation from cubic to hexagonal geometry in the Ni-rich surface during the reaction. This transformation is kinetically inaccessible when starting from the La-rich surface.
Technologically tuning the surface composition to unlock a particularly active transformation pathway offers an attractive additional avenue for future electrocatalyst research and exploitation. Further development of local-structure-sensitive operando probes of the electrocatalyst surface layer will be needed for unequivocal identification of such transformations.
Contact: Slavomír Nemšák
Researchers: C. Baeumer (Stanford University, SLAC National Accelerator Laboratory, and Aachen University, Germany); J. Li and M. Bajdich (SLAC); Q. Lu (Stanford, SLAC, and ALS); A.Y.-L. Liang, J.T. Mefford, and W.C. Chueh (SLAC and Stanford); L. Jin, T. Duchoň, M.A. Wohlgemuth, M. Giesen, R. Dittmann, and F. Gunkel (Forschungszentrum Jülich, Germany); H.P. Martins (ALS); M. Glöß (Forschungszentrum Jülich and Leibniz-Institute of Surface Engineering, Germany); S.M. Gericke (Berkeley Lab); E.E. Penn (Stanford); R. Waser (Forschungszentrum Jülich and Aachen University); and S. Nemšák (ALS and Forschungszentrum Jülich).
Funding: Horizon 2020 (European Union research and innovation program); U.S. Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES); German Science Foundation; and National Science Foundation. Operation of the ALS is supported by DOE BES.
Publication: C. Baeumer, J. Li, Q. Lu, A.Y.-L. Liang, L. Jin, H.P. Martins, T. Duchoň, M. Glöß, S.M. Gericke, M.A. Wohlgemuth, M. Giesen, E.E. Penn, R. Dittmann, F. Gunkel, R. Waser, M. Bajdich, S. Nemšák, J.T. Mefford, and W.C. Chueh, “Tuning electrochemically driven surface transformation in atomically flat LaNiO3 thin films for enhanced water electrolysis,” Nat. Mater. 20, 674 (2021), doi:10.1038/s41563-020-00877-1.
Adapted from the Berkeley Lab news release, “A 1-Atom-Deep Look at a Water-Splitting Catalyst” and the SLAC National Accelerator Laboratory press release, “Study Shows Tweaking One Layer of Atoms on a Catalyst’s Surface Can Make It Work Better.”
ALS SCIENCE HIGHLIGHT #440