SCIENTIFIC ACHIEVEMENT
With the help of structural insights from the Advanced Light Source (ALS), researchers optimized the fit between organic and inorganic ions in a perovskite solar-cell material.
SIGNIFICANCE AND IMPACT
The work increased the material’s power-conversion efficiency and stability and opens up a new avenue for improving the current-carrier dynamics of a promising class of materials.
A photovoltaic rising star
To address the effects of global climate change, it’s essential that we capitalize on energy from the sun. However, although solar energy is freely available, it needs to be converted into usable electricity in a way that’s efficient, cost-effective, and commercially scalable.
Perovskites are high-performance inorganic semiconductors recognized as some of the most promising photovoltaic materials of the future. Perovskite films—thin, lightweight, and flexible—can be produced using low-cost solution-processing techniques, and their power-conversion efficiencies (PCEs) have rapidly risen to the brink of 30% in just 15 years, surpassing conventional silicon panels.
A structure with room to tinker
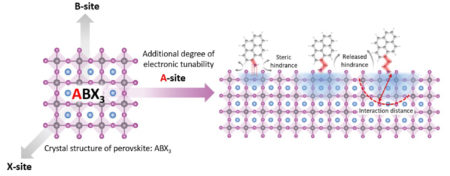
The most intriguing perovskite materials today are organic–inorganic hybrids. They have the general formula ABX3, in which the inorganic B and X ions form a framework of octahedral cages, and the organic A ions are located in the spaces between the cages.
Previously, it was thought that perovskite electronic performance mainly depended on the B and X electronic orbitals, and that A merely served a structural function. In this work, researchers showed that A-site organic ions with specially designed characteristics can increase charge-carrier mobility and power conversion efficiency while also improving device stability.
Trying ions on for size
The samples in this study were metal–halide perovskites in which B was lead (Pb2+) and X was iodide (I–). The organic component A consisted of ammonium (NH4+) attached to a “tail’ of pyrene (a four-ringed hydrocarbon), with three different lengths of hydrocarbon chain tethering the pyrene to the ammonium (PRA, PRMA, and PREA, in order of increasing chain length). When these organic variants were assembled with the inorganic framework, the extent to which the positively charged ammonium intercalated into the octahedral cavities depended on the length of the bridging chain.
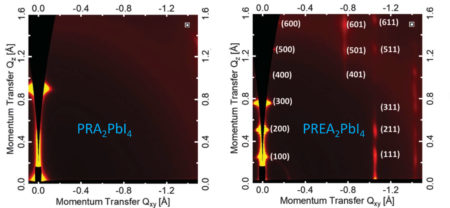
At ALS Beamline 7.3.3, the researchers performed grazing-incidence wide-angle x-ray scattering (GIWAXS) experiments on perovskite films that were surface-treated with ammonium to understand how the pyrene-based ammonium ions influence the perovskite structure.
Organic–inorganic orbital overlap
The structural data, first-principles calculations, and measurements of photoinduced charge-carrier mobility enabled the researchers to unravel the underlying mechanism of the A-site organic-ion modifications. Electron delocalization in the pyrene structure (due to overlapping p orbitals) enabled the A-site ions to contribute electronic states to the perovskite valence band. The PREA sample in particular (with the longest chain) appeared to have the optimal intercalation characteristics, exhibiting increased hole mobility and PCE as well as improved stability. Moreover, the beneficial effect of the pyrene tail on carrier mobility was also observed in an all-inorganic perovskite, indicating the generality of the concept.
Thus, control of the intercalation distance, regulated by the length of the hydrocarbon tether, provided an additional degree of electronic tunability that enables specially designed A-site ions to enhance the perovskite’s electronic performance. The work revises earlier assumptions about which molecular components affect the electronic performance of hybrid perovskites and opens up a new research direction in understanding and improving the carrier dynamics of perovskite photovoltaic devices.
Contacts: Yang Yang and Chenhui Zhu
Researchers: J. Xue, R. Wang, T. Huang, Y. Zhao, D. Meng, S. Tan, and Y. Yang (Univ. of California, Los Angeles); X. Chen, Y. Zhai, K. Zhu, and M.C. Beard (National Renewable Energy Laboratory); C. Yao and Y. Yan (Univ. of Toledo); X. Jin and R. Liu (Yangzhou Univ., China); K.-L. Wang and Z.-K. Wang (Soochow Univ., China); W. Huang (Monash Univ., Australia); and C. Zhu (ALS).
Funding: U.S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Solar Energy Technologies Office; Priority Academic Program Development of Jiangsu Higher Education Institutions; Natural Science Foundation of China; Collaborative Innovation Center of Suzhou Nano Science and Technology. Operation of the ALS is supported by the U.S. DOE, Office of Science, Basic Energy Sciences program.
Publication: J. Xue, R. Wang, X. Chen, C. Yao, X. Jin, K.-L. Wang, W. Huang, T. Huang, Y. Zhao, Y. Zhai, D. Meng, S. Tan, R. Liu, Z.-K. Wang, C. Zhu, K. Zhu, M.C. Beard, Y. Yan, and Y. Yang, “Reconfiguring the band-edge states of photovoltaic perovskites by conjugated organic cations,” Science 371, 636 (2021), doi:10.1126/science.abd4860.
ALS SCIENCE HIGHLIGHT #443