Assessing the environmental and public health impacts of the Deepwater Horizon oil spill is difficult owing to the extreme depth of the blowout and the large volumes of oil released. One strategy for remediation of the plume is to use the intrinsic bioremediation potential of deep-sea microorganisms to degrade the oil. This strategy depends on a number of environmental factors, including a favorable response of indigenous microorganisms to an increased concentration of hydrocarbons and/or dispersant. To study the effects of the spill, researchers collected deep-water samples from across the Gulf of Mexico and analyzed their physical, chemical, and microbiological properties using a variety of techniques, including synchrotron radiation Fourier-transform infrared (SR-FTIR) spectroscopy at ALS Beamline 1.4. The studies suggest that the plume did indeed stimulate indigenous deep-sea bacteria that are closely related to known petroleum degraders.

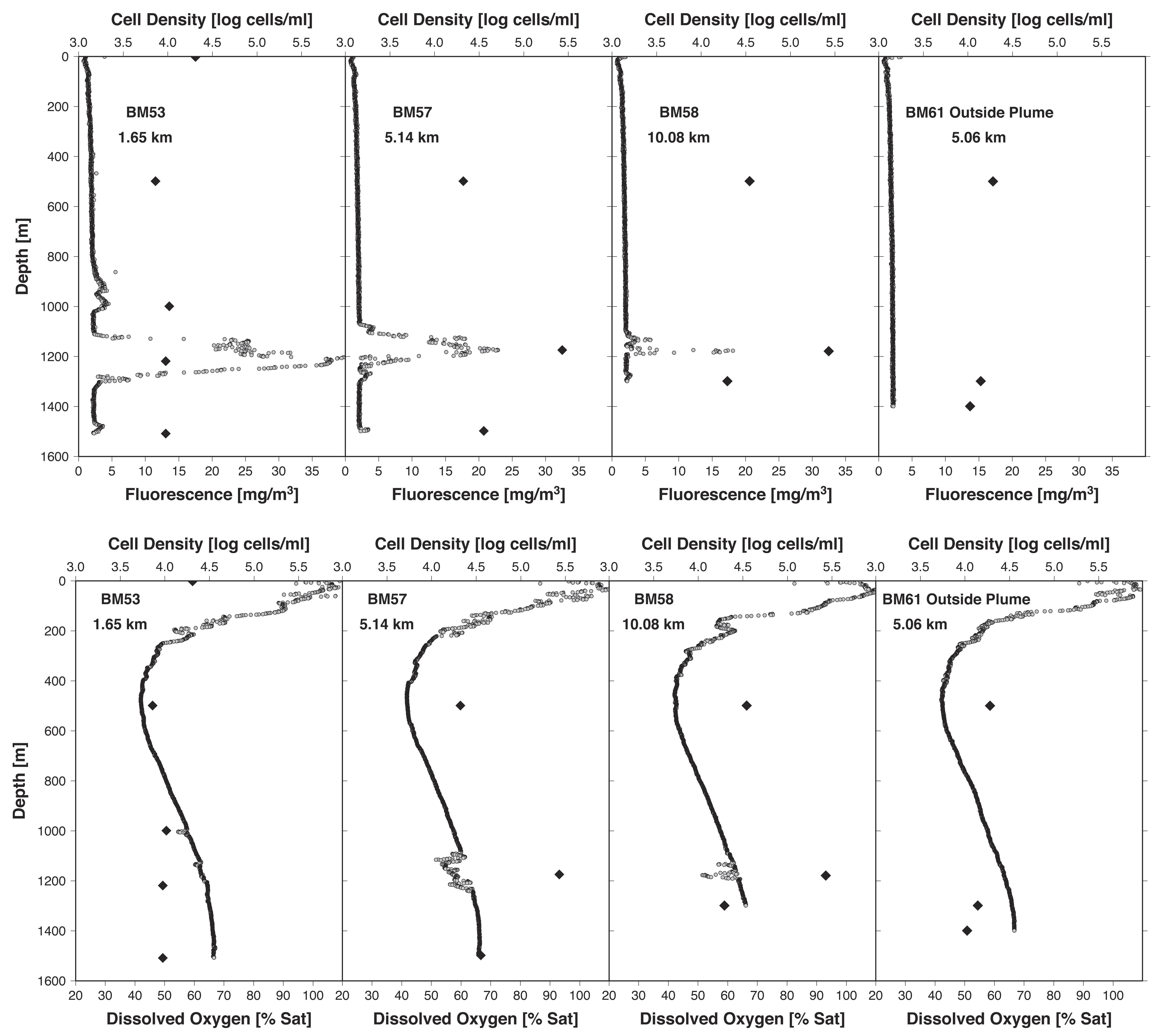
Seventeen deep-water samples were taken between May 25 and June 2, 2010, from across the Gulf of Mexico. The data indicated the presence of an oil plume from 1099 to 1219 m deep at distances of up to 10 km from the wellhead with a southwest current. The average temperature within the plume was 4.7°C and the pressure was 1136 dB (1 atm = 191 dB). At most plume locations, a slight decrease in oxygen concentration was detected, indicative of microbial respiration and oxygen consumption, as would be expected if the hydrocarbons were being catabolized. Molecular biochemistry analyses revealed that the dispersed oil plume affected microbial cell densities and composition. Cell densities inside the plume were almost two times higher than outside the plume.
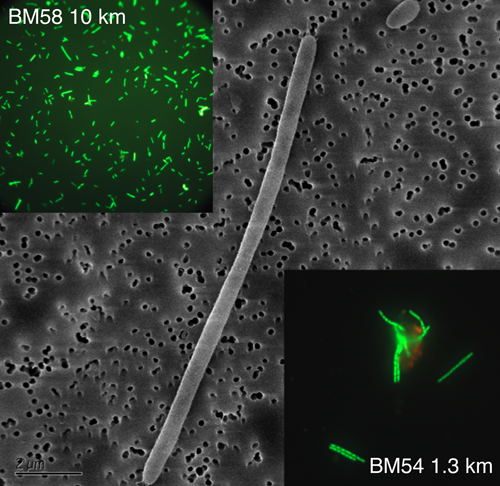
Genetic analysis of in-plume samples revealed that, while cell densities were higher, taxonomic diversity was lower. The enriched bacteria were restricted to a few members of the γ-Proteobacteria subphylum, including psychrophilic and psychrotolerant (cold-water thriving or tolerant) species. Further cloning and sequencing revealed that the samples were dominated by the order Oceanospirillales, with the observed sequences falling into one of two distinct Oceanospirillales groups. One of these groups is largely composed of known psychrophilic hydrocarbon degraders and microorganisms from hydrocarbon-dominated environments, including Oleispira antarctica, Thalassolituus oleivorans (psychrophiles),and Oleiphilus messinensis, all of which are known hydrocarbon degraders. Microscopic examination of cells collected within the plume also revealed that the dominant cell type exhibits a distinctive morphology typical of the Oceanospirillales.
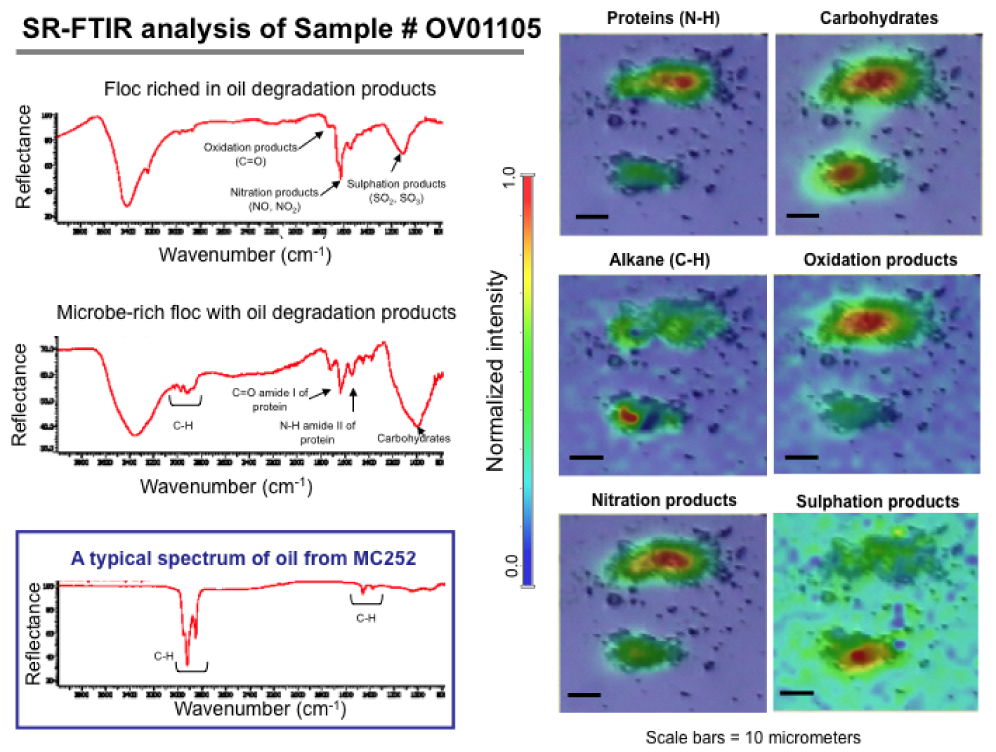
Using SR-FTIR data collected at infrared Beamline 1.4, the researchers found absorption features at ~1730, ~1610, and ~1150 cm-1 that are associated with biomolecule-rich regions. The mid-infrared light (~2.5- to 15.5-m wavelength or ~4000- to 650-cm-1 wavenumber) from the beamline is ideal for studying living bacteria individually or in small groups. This light is nondestructive and provides label-free, fingerprint-like spectra originating from the characteristic vibrational frequencies of various functional-group molecules. The absorption features from the samples are well described for the carbonyl (C=O), nitrogen oxide (NOx), and sulfur oxide (SOx) vibration modes, and they are characteristic of oil degradation products as well as macromolecules of biological samples. These infrared spectra are not consistent with those typically found in marine macroaggregates. Most importantly, we do not observe them in the nonplume samples at the same depth.
The bioremediation potential of these microorganisms largely depends on how fast they degrade the oil. Based on the biodegradation rates from four data sets (two from the field and two from the laboratory), the researchers predicted that the oil half-life is somewhere between 1.2 to 6.1 days. This is consistent with values reported in the literature for comparable temperature and field conditions. The frequent episodic oil leaks from natural seeps in this area suggest that the deep-sea microbial community may have adapted to fluctuating concentrations of hydrocarbons over long periods of time.
Contact: Terry Hazen
Research conducted by: T.C. Hazen, E.A. Dubinsky, T.Z. DeSantis, G.L. Andersen, Y.M. Piceno, N. Singh, J.R. Jansson, A. Probst, S.E. Borglin, J.L. Fortney, M. Bill, M.S. Conrad, L.M. Tom, K.L. Chavarria, T.R. Alusi, R. Lamendella, D.C. Joyner, M. Auer, M.L. Zemla, R. Chakraborty, E.L. Sonnenthal, H.-Y. N. Holman, S. Osman, and O. U. Mason (Berkeley Lab); W.T. Stringfellow (Berkeley Lab and University of the Pacific); C. Spier (University of the Pacific); P. D’haeseleer (Lawrence Livermore National Laboratory); Z.Lu, J.D. Van Nostrand, and Y. Deng (University of Oklahoma); and J. Zhou (Berkeley Lab and University of Oklahoma).
Research funding: Energy Biosciences Institute, University of California, Berkeley; University of Oklahoma Research Foundation. Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: T.C. Hazen, E.A. Dubinsky, T.Z. DeSantis, G.L. Andersen, Y.M. Piceno, N. Singh, J.R. Jansson, A. Probst, S.E. Borglin, J.L. Fortney, W.T. Stringfellow, M. Bill, M.S. Conrad, L.M. Tom, K.L. Chavarria, T.R. Alusi, R.Lamendella, D.C. Joyner, C. Spier, M. Auer, M.L. Zemla, R. Chakraborty, E.L. Sonnenthal, P. D’haeseleer, H.-Y.N. Holman, S. Osman, Z. Lu, J.D. Van Nostrand, Y. Deng, J. Zhou, and O.U. Mason, “Deep-sea oil plume enriches psychrophilic oil-degrading bacteria,” Science 330, 204 (2010).
ALS SCIENCE HIGHLIGHT #219