If you combine two substances and the resulting mixture has a lower melting point than either constituent alone, the system is “eutectic.” In a deep eutectic solvent (DES), the dip in melting point is more pronounced, and certain physical properties—such as viscosity, heat capacity, and molecular polarity—can be tuned by varying the relative mix of the parent constituents. Such changes affect the complex network of hydrogen bonds that is crucial to DES performance, not just as solvents, but in applications such as thermal energy storage and greenhouse-gas capture.
However, despite their ready tunability and industrial promise, there have been few x-ray spectroscopy studies of DES liquid/vapor interfaces, which are relevant to gas-capture applications. In this study, researchers probed the liquid/vapor interface of a benchmark DES called glyceline—a mixture of glycerol and choline chloride (ChCl)—using complementary spectroscopies at the Advanced Light Source (ALS).
“I’m interested in hydrogen bonding—for example, how hydrogen bonds get enhanced or disrupted,” said Musa Ahmed, a senior staff scientist in Berkeley Lab’s Chemical Sciences Division and principal investigator of the study. “My group has a whole body of work using multimodal x-ray spectroscopies, coupled with lasers and other lab-based tools, to look at hydrogen bonding.”
At the ALS, the researchers used ambient-pressure x-ray photoelectron spectroscopy (APXPS) at Beamline 9.3.2 to obtain depth profiles of the chemical species near the glyceline surface. A supersaturated (5:1) ChCl:Gly mixture enabled the sample to adhere to the vertical sample platform. To explore the effect of adding water, the researchers used velocity-map imaging (VMI) x-ray photoelectron spectroscopy at Beamline 9.0.1. This technique is capable of measuring molecular distribution at the surface of glyceline–water aerosols.
The APXPS results showed a doubling of choline cation (Ch+) concentration at the liquid/vapor interface. VMI indicated that the addition of water resulted in the depletion of Ch+ from the surface and the rearrangement of hydrogen bond structures. In addition, lab-based Raman spectroscopy revealed changes in Ch+ conformation as a function of mixing ratio. Overall, the techniques provide a complementary view of the liquid/vapor interface that can assist with the rational design of optimized DESs.
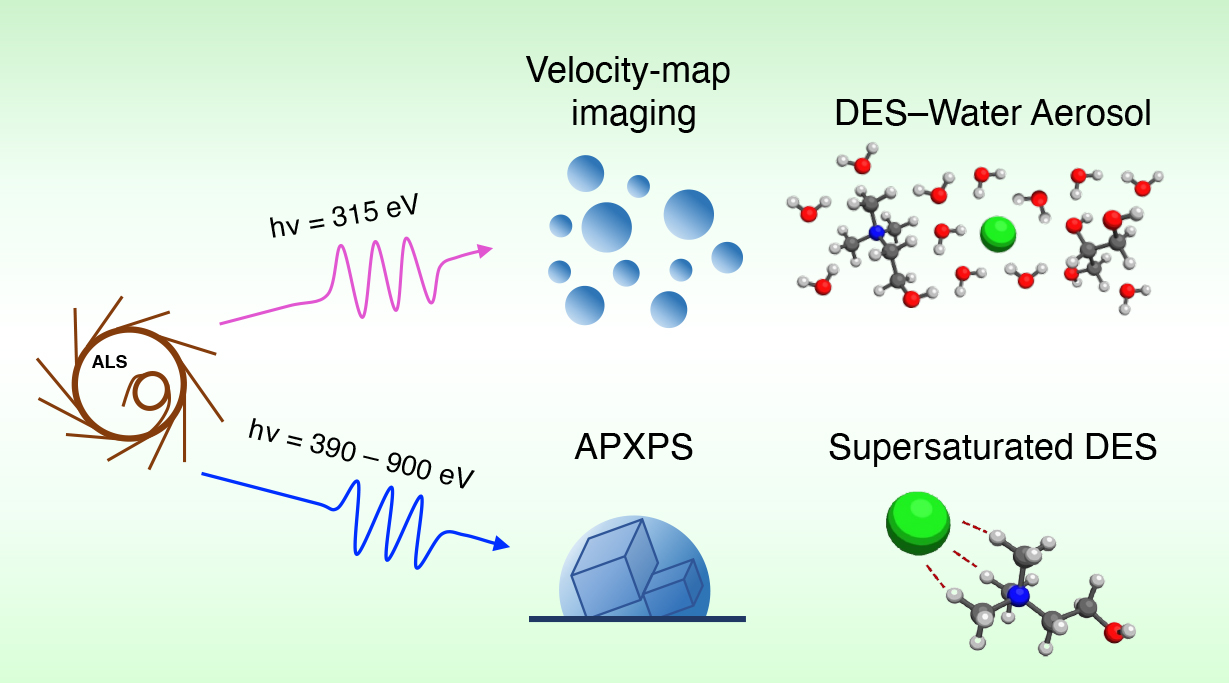
P. Kim, C. Weeraratna, S. Nemsak, N. Dias, A.K. Leemens, K.R. Wilson, and M. Ahmed, “Interfacial Nanostructure and Hydrogen Bond Networks of Choline Chloride and Glycerol Mixtures Probed with X-ray and Vibrational Spectroscopies,” J. Phys. Chem. Lett. 15, 3002 (2024), doi:10.1021/acs.jpclett.4c00052.