Organic aerosols (nanometer-sized liquid or solid particles suspended in air) are important constituents of the troposphere, and their chemistry has large-scale impacts on climate, pollution, and health. Accurate predictions of these aerosol impacts require a robust microphysical understanding of all relevant chemical reaction mechanisms and time scales, including those involving highly reactive free-radical molecules. However, detailed modeling is complicated by the large number of possible chemical transformations that aerosols undergo in the atmosphere. To help clarify the situation, researchers from Berkeley Lab measured multiphase aerosol reaction rates using the vacuum-ultraviolet (VUV) aerosol mass spectrometer (AMS) at the Chemical Dynamics Beamline of the ALS. They discovered an unexpectedly large acceleration in aerosol oxidation in the presence of anthropogenic pollutants commonly found in smoggy air, a result that could help bring models closer in line with observations.

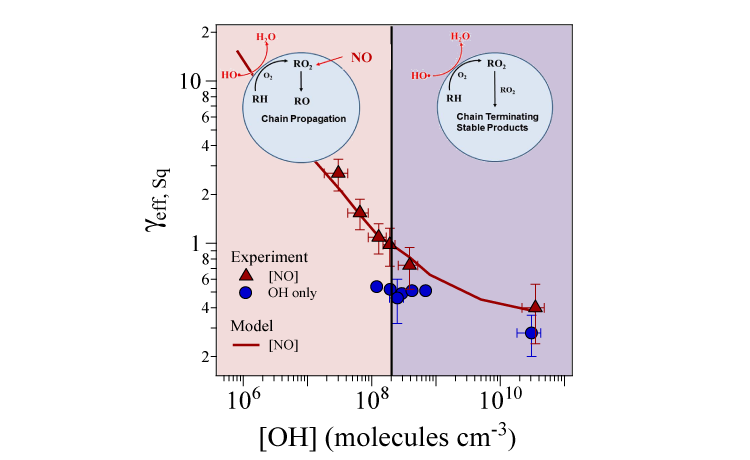
In this work, heterogeneous (gas/liquid or gas/solid) reaction rates were measured in “polluted” atmospheres, i.e. in the presence of gas-phase nitric oxide (NO), on single-component organic-aerosol proxies (both liquid or solid) in a specialized reaction chamber designed to mimic atmospheric conditions. The chemical compositions of the proxies were measured using a VUV-photoionization aerosol mass spectrometer at ALS Beamline 9.0.2. The tunability of the VUV light allowed the photoionization energy to be tuned to the ionization threshold of each molecule, reducing the degree of molecular fragmentation (a form of noise in the spectrum) and simplifying the analysis of the spectral fingerprints of the product molecules. The rate of decay of a given proxy was used to quantify the effective uptake coefficient, γeff, which in turn revealed how fast a reaction occurred. The results show that, as the OH concentration decreases, there is a steep increase in the effective uptake of the aerosol.
Because OH quickly reacts with nearby hydrocarbons and oxygen to produce peroxy radicals (RO2, where R is a generic hydrocarbon-based radical), at high concentrations of OH, the concentration of RO2 will also be relatively high. Thus, at high OH concentrations, RO2 radicals mostly react with other RO2 radicals, which leads to stable products that do not react any further. At much lower OH concentrations (approaching atmospheric levels), RO2 reacts primarily with NO, which leads to sustained, free-radical chain reactions that result in oxidation rates 10–30 times faster than previously observed.
These rate measurements, covering four orders of magnitude in OH concentration, overturn the commonly held belief that gas-phase reactions dominate organic aerosol formation and aging and that heterogeneous oxidation is simply too slow (on the order of weeks) to play a significant role in the daily chemical transformations (on the order of hours) of organic aerosols in urban environments. The incorporation of this new free-radical chain-reaction mechanism into global and regional models is expected to improve aerosol mass and composition predictions.
Upcoming work will investigate the analogous role of SO2, also present in polluted environments, in accelerating the γeff heterogeneous oxidation rates of organic particles. Also, the researchers plan to evaluate the importance of NO in generating RO radicals in more complex aerosol systems and for more dilute aqueous solutions and semi-solids in order to better understand the role that molecular structure and particle phase has on chain cycling reactions.
Research conducted by: N.K. Richards-Henderson and K.R. Wilson (Berkeley Lab) and A.H. Goldstein (University of California, Berkeley).
Research funding: U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES) and Office of Science Early Career Research Program. Operation of the ALS is supported by DOE BES.
Publication about this research: N.K. Richards-Henderson, A.H. Goldstein, and K.R. Wilson, “Large Enhancement in the Heterogeneous Oxidation Rate of Organic Aerosols by Hydroxyl Radicals in the Presence of Nitric Oxide,” J. Phys. Chem. Lett.6, 4451 (2015).
ALS SCIENCE HIGHLIGHT #327