Experiments at the ALS have helped to explain how a new catalyst significantly boosts the formation of light olefin molecules—important building blocks in the petrochemical industry—from a basic gas mixture called syngas (synthesis gas). A research team from China recently developed the nanocomposite catalyst and used ambient-pressure x-ray photoelectron spectroscopy (AP-XPS) to investigate the chemical changes occurring at the catalytic surface in the presence of various reaction gases. The results showed that the boost in olefin production is enabled by the bifunctionality of the catalyst, which affords two types of active sites with complementary properties. The research should be of interest to both academia and industry, and the new technique could become a serious competitor for many older industrial processes, allowing for the use of alternative syngas feedstocks that can save water and energy.
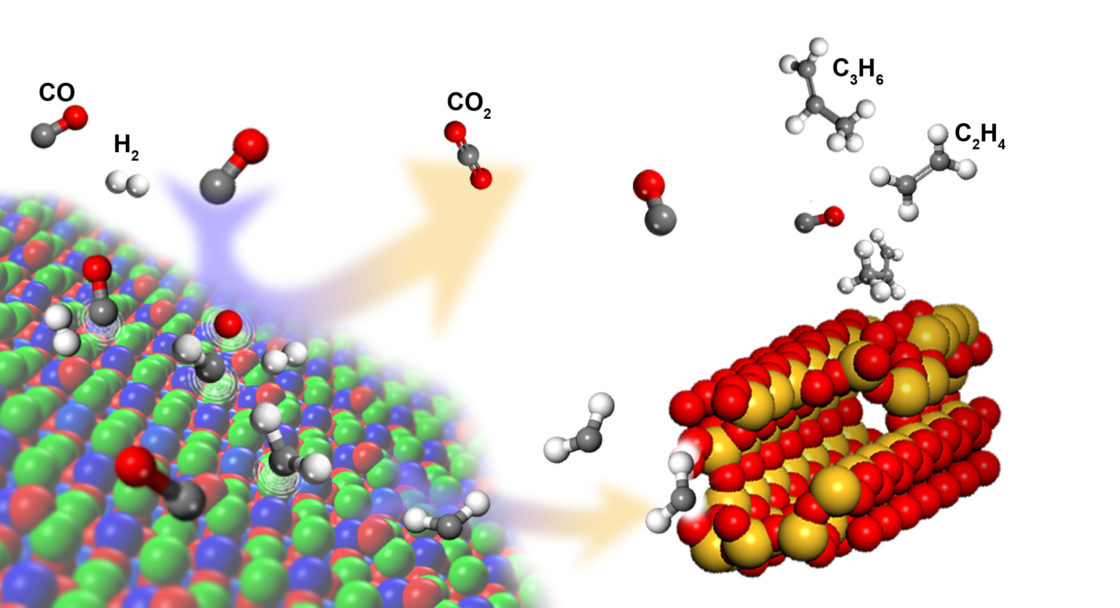
Syngas (a mixture of H2 and CO gases) can be converted into light olefins and other hydrocarbons through Fischer-Tropsch synthesis (FTS), a long-standing gas-to-liquid process involving metal-based catalysts. In FTS, H2 and CO are catalyzed to form CHx monomers. These monomers then undergo polymerization on the catalyst’s metal surface, leading to the formation of a wide range of hydrocarbons of different chain lengths. The percentage of a given hydrocarbon species produced (selectivity) depends on variables such as temperature, syngas composition (ratio of H2 to CO), pressure, catalyst type, and catalyst composition. A widely used statistical model of the process predicts that the maximum selectivity for hydrocarbons with two to four carbon atoms (C2–C4 hydrocarbons), including olefins, is around 58%.
Now, researchers from the Dalian Institute of Chemical Physics in China have developed a nanocomposite catalyst consisting of a metal oxide (ZnCrOx) and a mesoporous zeolite (a mineral with acidic pores). With this catalyst, they were able to achieve a surprisingly high selectivity of 80% for light olefins and 94% more generally for C2–C4 hydrocarbons. Syngas with a lower ratio of H2 to CO (such as coal- or biomass-derived syngas) can be used, avoiding the need to increase the proportion of H2 via the water-gas shift reaction, an energy-intensive and water-consuming process.
To better understand the functionality of the catalyst, the team carried out in situ ambient-pressure x-ray photoelectron spectroscopy (AP-XPS) measurements of the ZnCrOx surface, working with scientists at Beamline 9.3.2 of the ALS, where the AP-XPS technique was pioneered. The resulting spectra indicate that the surface undergoes partial reduction (removal of oxygen) upon exposure to H2 and CO, leading to the formation of CO2 and surface carbon species. This latter carbon deposition, which might otherwise limit catalyst lifetime, can be removed by exposure to H2 to form the hydrocarbon building block, CH2. The AP-XPS data is consistent with characterization results from other methods such as time-of-flight mass spectrometry and density functional theory calculations.
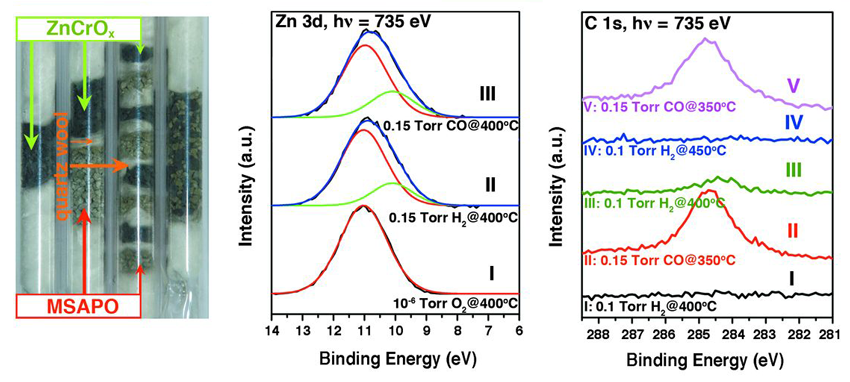
The overall reaction mechanism that emerges for this catalyst has two distinct stages, occurring at different functional sites. First, CO activation on the ZnCrOx surface leads to the formation of gas-phase intermediate hydrocarbon species (CH2). Then, the linking up of the intermediates into longer hydrocarbon chains (C–C coupling) takes place within the confines of the acidic (H+-donating) pores of the zeolite, where the length of the chains can generally be controlled by the size and acidity of the pores. Because the catalyst allows the use of syngas with a lower ratio of H2 to CO, the energy-intensive water-gas-shift reaction can be avoided. More generally, these findings open up promising new avenues for the development of syngas-to-olefin technology.
Contact: Xiulian Pan
Research conducted by: F. Jiao, Jinjing Li, X. Pan, J. Xiao, H. Li, H. Ma, M. Wei, M. Li, S. Miao, Jian Li, Y. Zu, D. Xiao, T. He, J. Yang, Q. Fu, and X. Bao (Dalian Institute of Chemical Physics, Chinese Academy of Sciences); Y. Pan, Z. Zhou, and F. Qi (National Synchrotron Radiation Laboratory, University of Science and Technology of China).
Research funding: National Natural Science Foundation of China, Ministry of Science and Technology of China, Chinese Academy of Sciences, and Dalian Institute of Chemical Physics Fundamental Research Program for Clean Energy. Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: F. Jiao, Jinjing Li, X. Pan, J. Xiao, H. Li, H. Ma, M. Wei, Y. Pan, Z. Zhou, M. Li, S. Miao, Jian Li, Y. Zu, D. Xiao, T. He, J. Yang, F. Qi, Q. Fu, and X. Bao, “Selective conversion of syngas to light olefins,” Science 351, 1065 (2016).
ALS SCIENCE HIGHLIGHT #335