In the 30 years since the discovery of the classic fullerene “buckyball” of 60 carbon atoms, scientists have found many variations on the fullerene theme: carbon nanotubes (elongated buckyballs), endohedral fullerenes (buckyballs with molecules inside), and even carbon “peapods” (nanotubes with buckyballs inside). Within this great diversity, however, certain rules emerged. One such rule governed the number and placement of the pentagonal and hexagonal carbon rings that are the basic fullerene building blocks. Now, scientists have used small-molecule x-ray crystallography at the ALS to verify and characterize a new rule-breaking fullerene that incorporates a seven-membered (heptagonal) carbon ring. This new molecule changes the definition of a classical fullerene and expands the range of structural possibilities for endohedral fullerenes, which have desirable physical properties with applications in solar cells, image contrasting, and medicine.
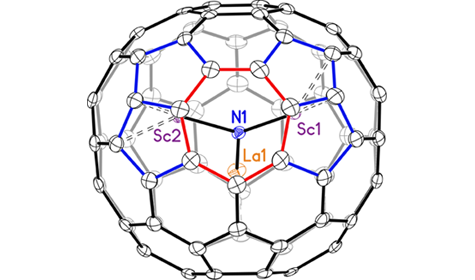
Fullerenes are a unique form of carbon consisting of molecular cages (two other forms, or allotropes, of carbon are diamond and graphite). This class of molecular cages can contain atoms inside (indicated by the symbol “@” in the fullerene name), or they can react with other molecules that bind to the surface of the cage. Both changes can affect the physical properties of the complex as a whole. Endohedral fullerenes with an interior cluster of the form M3N (M = metal, N = nitrogen) and an 80-carbon cage typically possess highly symmetrical carbon cages, where the specific type of symmetry (the point group) is designated by the notation Ih or D5h. The first fullerene discovered in 1985, the iconic 60-carbon molecular soccer ball, is designated as Ih-C60. Fullerenes in the Ih and D5h point groups are isomers (molecules with same chemical formula but different structures) that obey the isolated pentagon rule (IPR), in which every pentagonal ring is surrounded by hexagonal rings. Their molecular symmetries and structures can be verified via single-crystal x-ray diffraction.
In this work, researchers identified a rule-breaking mixed-metal fullerene using small-molecule x-ray diffraction at ALS Chemical Crystallography Beamline 11.3.1. This fullerene, LaSc2N@Cs(hept)-C80, was prepared via arc discharge between graphite rods doped with La2O3 and Sc2O3. The results showed that LaSc2N@Cs(hept)-C80 breaks the rules that define classical fullerenes in that it contains a heptagon in its ring system, giving it a different point-group symmetry (Cs). Generally, fullerenes are composed of only pentagonal and hexagonal rings, which mathematically agrees with Euler’s theorem, according to which every fullerene contains 12 pentagons, with the remaining rings being hexagons. In the new fullerene, there are also two pentalene units that violate the IPR by having abutting pentagons. The presence of a heptagon and two pentalene units forces the structure to have an unprecedented 13 pentagons in total. Nevertheless, this molecule is still classified as a fullerene, since it consists of an all-carbon cage.
Two other isomers, LaSc2N@Ih-C80 and LaSc2N@D5h-C80, were also isolated. Historically, these are the two most common isomers of the M3N@C80 cage, with the Ih isomer being far more prevalent than D5h. The appearance of the Cs(hept) isomer was not anticipated. However, computational analysis for LaSc2N@C80 suggests that, while the Ih cage is lowest in relative energy, the Cs(hept) cage is only 27 kJ/mol less stable, and the Cs(hept) cage is 34 kJ/mol more stable than the D5h cage. Thus, previous failure to isolate the Cs(hept) cage may be ascribed to kinetic factors rather than to thermodynamic stability. The researchers proposed that a low yield of the Cs(hept) cage would arise if it has a low barrier to rearrangement into other isomers. Several different rearrangements are possible, all of which have a bearing on the still-unknown mechanism for the synthesis of multiple products in the plasma of the arc-discharge process. Now that the new heptagonal isomer has been characterized, the search is on for other examples.
Contact: Kamran Ghiassi
Research conducted by: Y. Zhang, Q. Deng, N.A. Samoylova, and A.A. Popov (Leibniz Institute for Solid State and Materials Research, Germany); and K.B. Ghiassi, M.M. Olmstead, and A.L. Balch (University of California, Davis).
Research funding: German Research Foundation (DFG) and National Science Foundation. Operation of the ALS is supported by the U.S. Department of Energy (DOE), Office of Basic Energy Sciences.
Publication about this research: Y. Zhang, K.B. Ghiassi, Q. Deng, N.A. Samoylova, M.M. Olmstead, A.L. Balch, and A.A. Popov, “Synthesis and Structure of LaSc2N@Cs(hept)-C80 with One Heptagon and Thirteen Pentagons,” Angew. Chem. Int. Ed. 54, 495 (2015).
ALS SCIENCE HIGHLIGHT #311