SCIENTIFIC ACHIEVEMENT
Using time-resolved experiments at the Advanced Light Source (ALS), researchers found a way to count electrons moving back and forth across a model interface for photoelectrochemical cells.
SIGNIFICANCE AND IMPACT
The findings provide real-time, nanoscale insight into the efficiency of nanomaterial catalysts that help turn sunlight and water into fuel through artificial photosynthesis.
Solar-fuel tech goes for gold
In the search for clean-energy alternatives to fossil fuels, one promising solution relies on photoelectrochemical (PEC) cells: water-splitting, artificial-photosynthesis devices that turn sunlight and water into solar fuels such as hydrogen. In just a decade, researchers have achieved great progress in the development of PEC systems made of light-absorbing gold nanoparticles (NPs) attached to a semiconductor film of titanium dioxide (TiO2).
Despite these advancements, researchers still struggle to make a device that can produce solar fuels on a commercial scale. To explain why some water-splitting PEC devices do not work as well as hoped, researchers have now quantified electron transfers between gold and TiO2 at the nanoscale and in real time. The results should help researchers develop more-efficient material combinations for high-performance solar-fuel devices.
A productive pairing comes to light
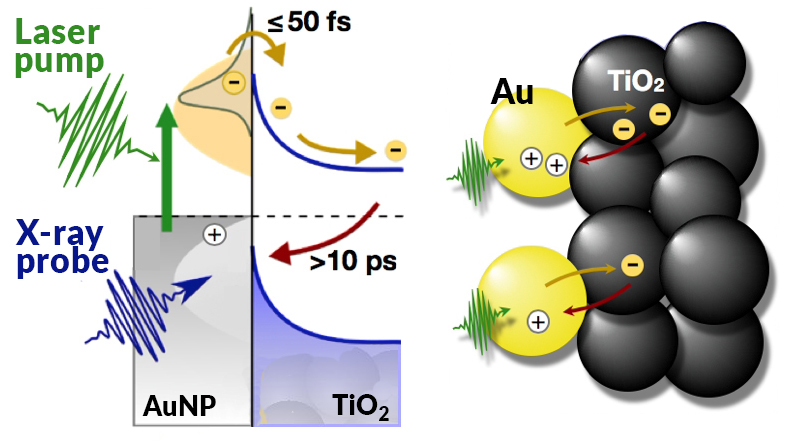
When illuminated by sunlight, electrons in gold NPs move in sync with the electric field of the light (“plasmonic resonance”). Some of the electrons pass through the gold/TiO2 interface, leaving holes behind. These electron–hole pairs are critical ingredients for enabling the chemical reaction that produces solar fuels. The longer the lifetime of the electron–hole pairs, the more time there is for the chemical reaction to take place.
Thus, to understand how efficiently a PEC device is working, it’s necessary to know the number of electrons moving between the gold and TiO2 as well as how long the electron–hole pairs last. By tracing the movement of electrons in these complex systems with chemical specificity and picosecond time resolution, an accurate calculation of the conversion efficiency of plasmonic light-harvesting devices can be obtained.
An electronic “count”-down
At ALS Beamline 11.0.2.1, researchers performed time-resolved, pump-probe x-ray photoelectron spectroscopy (XPS) experiments in which pulses of laser light were used to excite electrons in gold NPs on a TiO2 film. Delayed x-ray pulses then probed both the electron donor (Au) and acceptor (Ti) materials. Comparison of the excited- and ground-state spectra revealed a shift in the excited Au spectrum to higher binding energies, but no observable effect in the Ti.
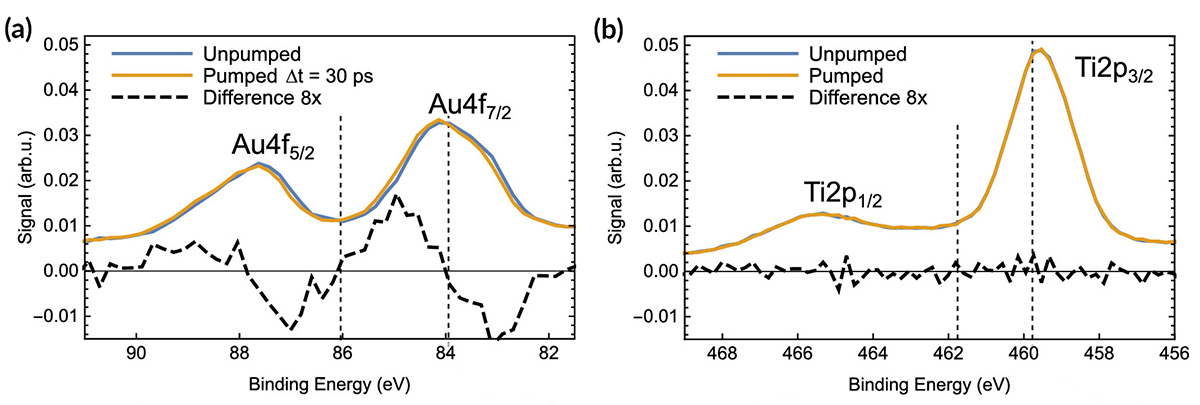
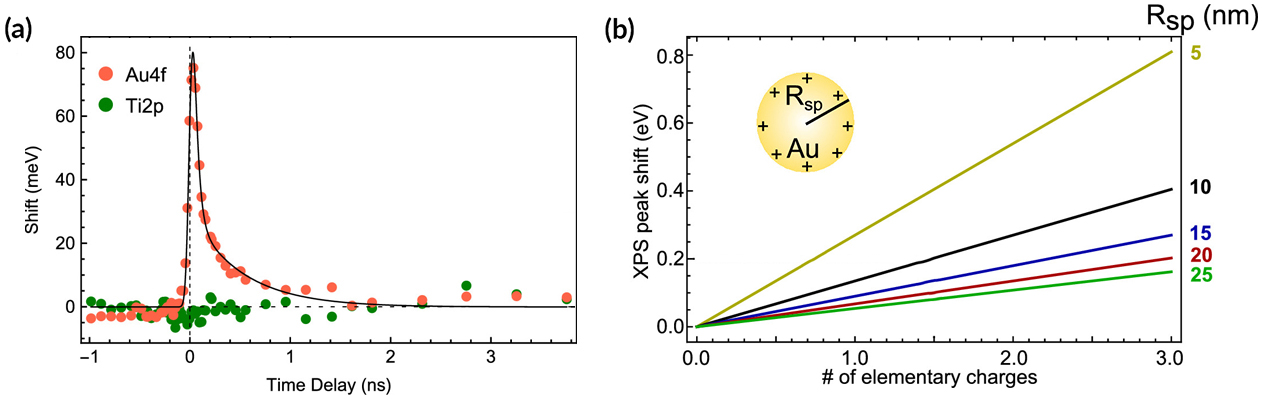
Analysis of the time dependence of the shift in Au spectra indicated a time scale of about 1 ns for the electron–hole pair lifetime. The number of electrons transferred was obtained by modeling the shift in binding energy as being due to a constant potential created by the accumulation of excess or missing charge at the NP surface. The model predicts that the number of electrons transferred is linearly dependent on the shift in binding energy. In this case, that meant approximately two electrons were transferred per NP—far fewer than expected. The corresponding photon-to-charge conversion efficiency was 0.1%.
Although XPS is commonly used around the world, this work is a unique expansion of the technique to time-resolved studies and is the first time it has been applied to charge-transfer dynamics in nanoplasmonic systems. The research team next plans to push their measurements to even faster time scales with a free-electron laser and to capture even finer nanoscale snapshots of electrons at work in a PEC device when water is added to the mix.
Contact: Oliver Gessner
Researchers: M. Borgwardt, J. Mahl, F. Brauße, G. Liu, F.M. Toma, and O. Gessner (Berkeley Lab); F. Roth (Technische Universität Bergakademie Freiberg); L. Wenthaus (Deutsches Elektronen Synchrotron); M. Blum (Berkeley Lab and ALS); and K. Schwarzburg (Helmholtz-Zentrum Berlin für Materialien und Energie).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES); Joint Center for Artificial Photosynthesis; and Alexander von Humboldt Foundation. Operation of the ALS is supported by DOE BES.
Publication: M. Borgwardt, J. Mahl, F. Roth, L. Wenthaus, F. Brauße, M. Blum, K. Schwarzburg, G. Liu, F.M. Toma, and O. Gessner, “Photoinduced Charge Carrier Dynamics and Electron Injection Efficiencies in Au Nanoparticle-Sensitized TiO2 Determined with Picosecond Time-Resolved X-ray Photoelectron Spectroscopy,” J. Phys. Chem. Lett. 11, 5476 (2020), doi:10.1021/acs.jpclett.0c00825.
Adapted from the Berkeley Lab news release, “Scientists Capture Candid Snapshots of Electrons Harvesting Light at the Atomic Scale.” See also the DOE highlight, “Watching Electrons Harvest Light at the Nanoscale.”
ALS SCIENCE HIGHLIGHT #427