Electrocatalysts are responsible for expediting reactions in many promising renewable energy technologies. However, the extreme sensitivity of their surface redox states to temperatures, to gas pressures, and to electrochemical reaction conditions renders them difficult to investigate by conventional surface-science techniques. Recently a team of Stanford and Berkeley Lab researchers used x-rays at the ALS in a novel way to observe the behavior of electrons during technologically important chemical reactions in metal oxide electrocatalysts. What they learned has upended long-held scientific understanding of how these catalysts work.
Many of today’s most promising renewable energy technologies—fuel cells, water splitters, and artificial photosynthesis—rely upon catalysts to expedite the chemical reactions. Catalysts are materials that enhance chemical reactions without being consumed in the process. Ceramic-like metal oxides, such as iron oxide (a material similar to rust) are desirable as catalysts because they are more abundant and more stable than typical catalysts made of rare metals like platinum, ruthenium and rhodium. Although they may be more abundant, however, metal oxides are also less scientifically understood than their metallic counterparts.
Electrochemical reactions involving oxygen gas molecules, in particular, are ubiquitous in energy conversion and storage technologies. A technologically- important class of oxygen electrochemical reactions is one that simultaneously reduces oxygen molecules to and incorporates them as solid-state oxygen ions and vice versa. At the ALS, researchers were able to directly observe redox processes in thin-film iron and cobalt perovskite oxide electrocatalysts using surface-sensitive, x-ray absorption spectroscopy while the electrochemical reactions took place.
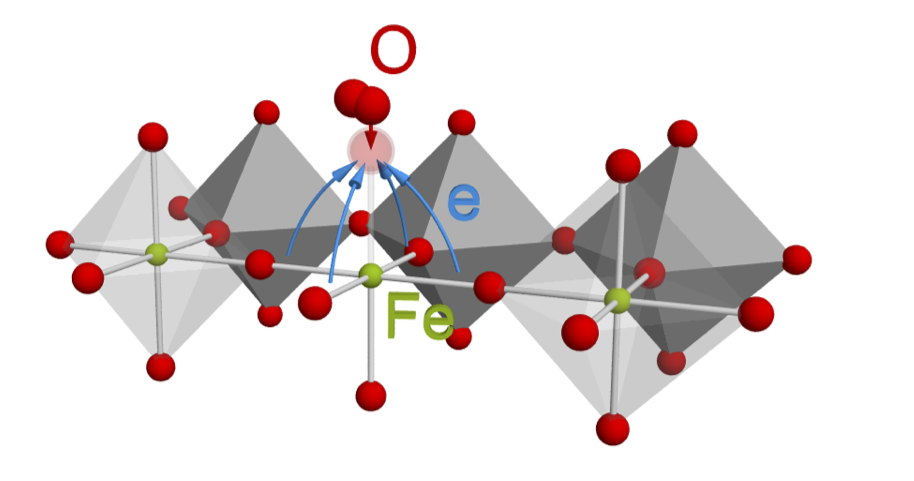
In contrast to the conventional view that the transition metal cations are the dominant redox-active centers, researchers found that the oxygen anions near the surface are a significant redox partner to molecular oxygen due to the strong hybridization between oxygen 2p and transition metal 3d electronic states. The research proposes that a narrow oxygen-ion electronic state exchanges electrons with the molecular oxygen. This result highlights the importance of surface anion-redox chemistry in transition-metal oxides electrocatalysts.
The findings could guide the search for new and better catalysts. Researchers are now looking to develop new catalysts by modifying the oxygen ions in these systems. Previously, it was challenging to study how gas molecules, particularly oxygen, interacted with the catalyst because researchers couldn’t include them in the experiments. Unique instruments at the ALS allowed the researchers to follow the electrons as they do their work and to better understand how they behave during the reactions. What they have learned is critical to fundamental-level understanding of catalysts and could lead to exciting new directions in catalyst design.
Contact: William Chueh
Research conducted by: D. N. Mueller and M. L. Machala (Stanford University), H. Bluhm (Chemical Sciences Division, LBNL), and W. C. Chueh (Stanford University, SLAC).
Research funding: U.S. Department of Energy (DOE), Basic Energy Sciences (BES). Operation of the ALS is supported by the DOE BES.
Publication about this research: D. N. Mueller, M. L. Machala, H. Bluhm, and W. C. Chueh, “Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions,” Nature Communications 6, 6097 (2015).
ALS SCIENCE HIGHLIGHTS #312