Cancer and autoimmune diseases pose contradictory challenges to our immune system. Because cancer cells originate within the body, the immune system does not always recognize their threat. In contrast, autoimmune diseases are characterized by an overactive immune system that attacks the body’s healthy cells.
The white blood cells of the human immune system include effector T cells, which are part of the immune system’s immediate response to foreign stimuli like viruses, and regulatory T cells, which suppress the immune response when it is no longer required. These two types of T cells participate in an autoimmune seesaw. When effector cells are activated, the immune system is better equipped to recognize the threat from cancer cells. When regulatory cells dominate, they can suppress autoimmune disease. Proposed therapies for cancer or autoimmune disease tip the seesaw using interleukin-2 (IL-2), a flexible signaling protein that activates by binding to the applicable cell type.
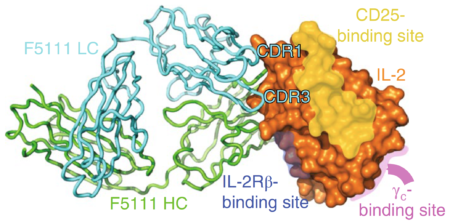
Using protein crystallography at ALS Beamline 8.2.1, researchers found a way to better control IL-2 signaling by identifying an antibody that locks it in a conformation that preferentially activates regulatory cells. By solving the crystal structure of IL-2 in complex with its antibody, the scientists discovered how IL-2 binds to the regulatory cell receptor, CD25. Once attached to CD25, IL-2 releases the antibody and proceeds to activate the regulatory cell population.
These results offer evidence for a human antibody that can be used in treating autoimmune diseases. This approach has also begun to inform the development of antibodies that activate effector T cells for cancer therapy.
E. Trotta, P.H. Bessette, S.L. Silveria, L.K. Ely, K.M. Jude, D.T. Le, C.R. Holst, A. Coyle, M. Potempa, L.L. Lanier, K.C. Garcia, N.K. Crellin, I.J. Rondon, and J.A. Bluestone, “A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism,” Nature Medicine 24, 1005–1014(2018), doi:10.1038/s41591-018-0070-2.