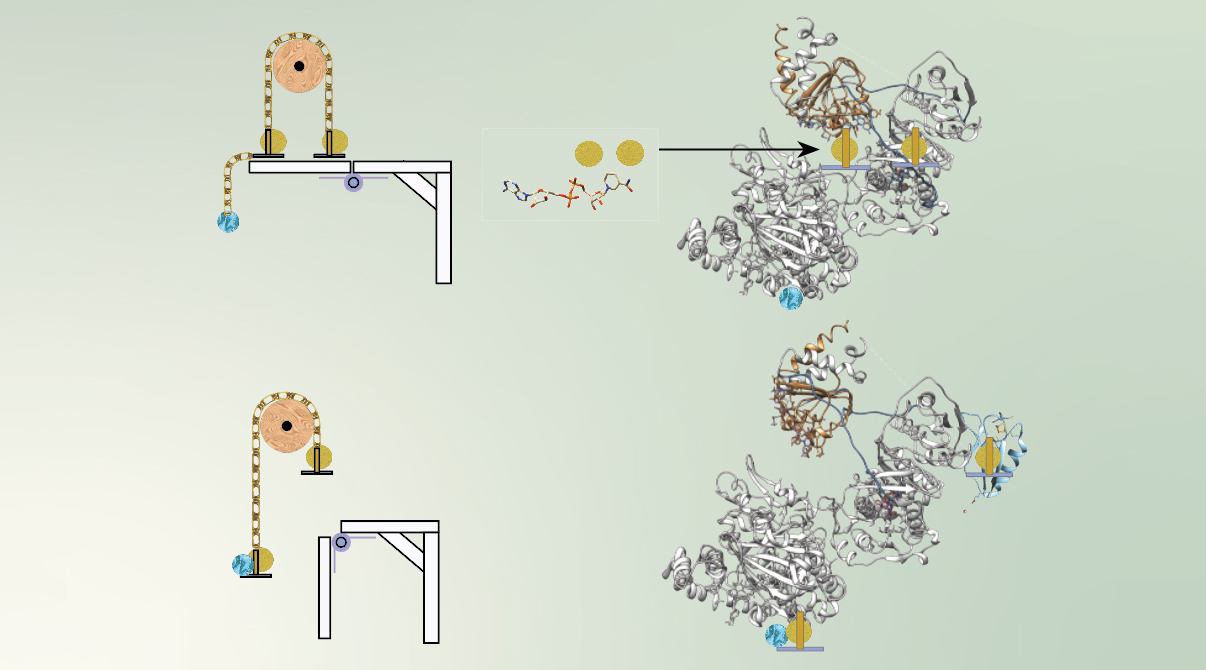
A class of chemical reaction found only in biology, electron bifurcation liberates two electrons from one donor and channels them to two separate acceptors. Analogous to a mechanical pulley system in which one weight rises as the other falls, one electron is elevated in energy at the expense of lowering the energy of the second. The higher-energy electron can then help perform high-energy work, such as bond-breaking or bond-making, as necessary for the organism’s survival. Now, a team of researchers has used small-angle x-ray scattering (SAXS) at the Advanced Light Source (ALS) to understand a microbial protein involved in this recently discovered bioenergetic pathway.
The protein in question, EtfABCX from the bacterium Thermotoga maritima, uses electron bifurcation to create a high-energy electron so that it can be loaded onto another protein called ferredoxin. Ferredoxin, used in many metabolic pathways that provide energy to organisms, facilitates electron transfer and depends on an iron–sulfur cluster integral to its structure to hold high-energy electrons. In this process, two electrons of medium potential get pulled off NADH, a type of B vitamin that serves as the electron donor.
One of the tricks EtfABCX employs is to couple electron transfer to conformational change. In experiments performed at ALS Beamline 12.3.1 (SIBYLS), the researchers studied the mechanism of electron channeling in the bifurcation process. Using SAXS, a solution-based technique that does not restrain motion, EtfABCX’s steps, triggers, and coordinated conformational changes were elucidated.
The researchers found that NADH activates the protein’s mechanics, allowing it to act as the wheel and ropes of the pulley system. Once electrons are removed, the protein occupies a new conformation that allows the hand-off of electrons between different regions of EtfABCX. The researchers identified motions in EtfABCX that help it perform its function and connected the observed motions to its catalytic cycle.
By following the mechanics and conformations of EtfABCX, the researchers were able to shed light on this novel biological mechanism where energetic electrons are channeled over relatively large distances. Understanding these novel bioenergetic systems will help build microbial energy flux models and aid in the annotation of newly discovered genes.
D.T. Murray, X. Ge, G.J. Schut, D.J. Rosenberg, M. Hammel, J.C. Bierma, R. Hille, M.W.W. Adams, and G.L. Hura, “Correlating conformational equilibria with catalysis in the electron bifurcating EtfABCX of Thermotoga maritima,” Biochemistry 63, 1 (2023), doi:10.1021/acs.biochem.3c00472.
Adapted from the Berkeley Biosciences Area highlight, “Researchers Leverage SAXS to Understand Aspect of Microbial Metabolism.”