Overexposure to sunlight, which is damaging to natural photosynthetic systems of green plants and cyanobacteria, is also expected to be damaging to artificial photosynthetic systems. Nature has solved the problem through a photoprotective mechanism called “nonphotochemical quenching,” in which excess solar energy is safely dissipated as heat. ALS researchers have recently discovered a surprising key event in this energy-quenching process.
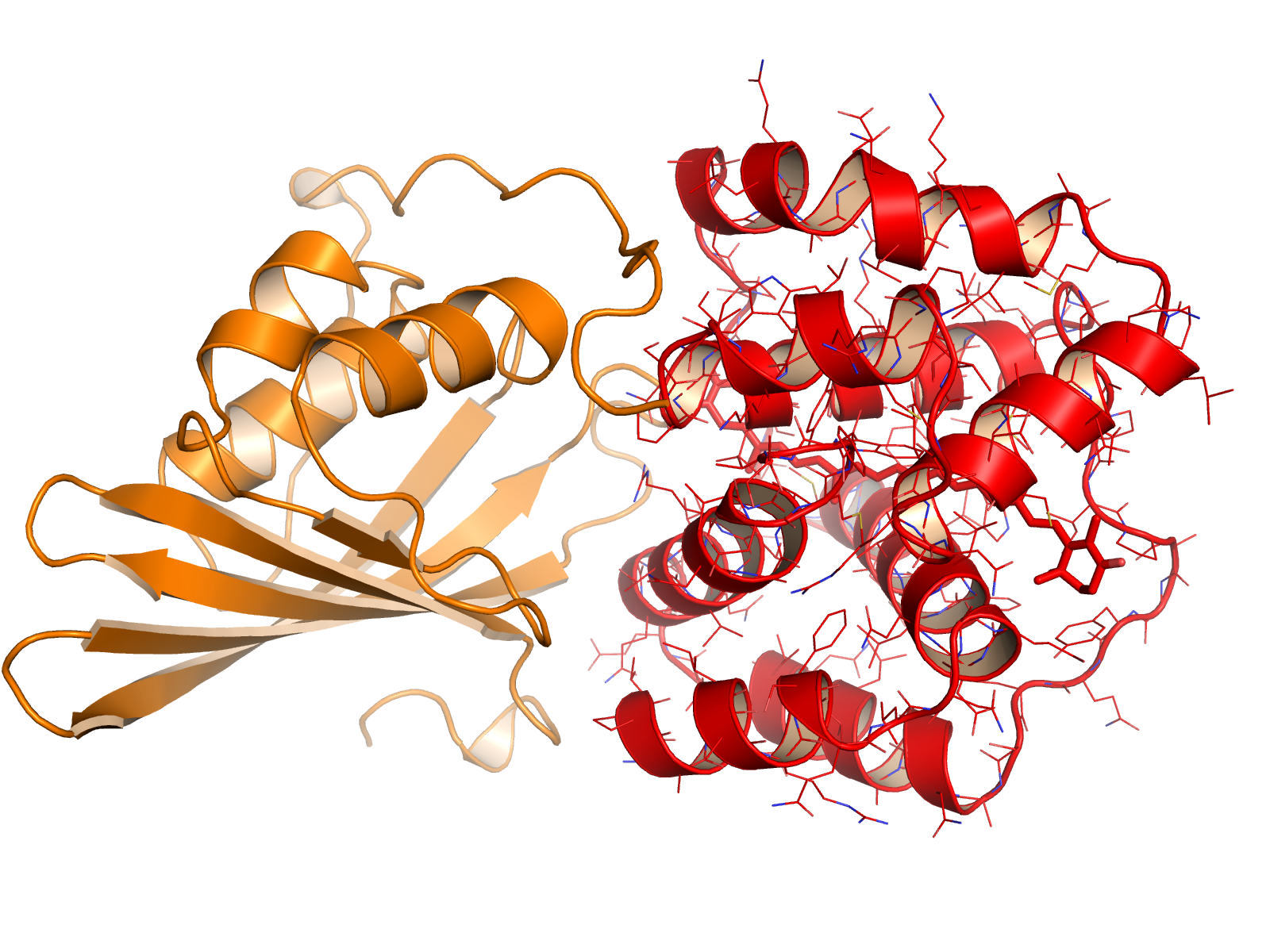
The research team found that in cyanobacteria the energy-quenching mechanism is triggered by an unprecedented, large-scale (relatively speaking) movement from one location to another of the carotenoid pigment within a critical light-sensitive protein called the Orange Carotenoid Protein (OCP). As a result of this translocation, the carotenoid changes its shape slightly and interacts with a different set of amino acid neighbors, causing the protein to shift from an “orange” light-absorbing state to a “red” photoprotective state. This turns out to be an unanticipated molecular priming event in photoprotection.
Prior to this work, the assumption was that carotenoids were static, held in place by the protein scaffold. Having shown that the translocation of carotenoid within the protein is a functional trigger for photoprotection, scientists are now looking to other carotenoid-binding protein complexes to see if translocation could play a role in those as well. Understanding the dynamic function of carotenoids could be useful for the design of future artificial photosynthetic systems.
As orange-state OCP absorbs blue-green light, it undergoes structural changes that result in red-state OCP. Once the excess solar energy has been quenched, the OCP interacts with a second protein, the Fluorescence Recovery Protein (FRP), causing it to revert back to the light-harvesting, orange state. Using the protein crystallography capabilities of ALS Beamline 5.0.2, researchers were able to obtain crystal structures of these key photoprotective proteins.
The crystal structures revealed that OCP photoactivation is accompanied by a 12 Å translocation of the pigment within the protein and a dramatic reconfiguration of carotenoid-protein interactions. The researchers also identified the origin of the photochromic changes in the OCP triggered by light and revealed the structural determinants required for interaction with the light-harvesting antenna during photoprotection.
To confirm that this translocation actually occurs when OCP is in its natural solution environment and was not due to structural changes that the protein undergoes during crystal formation, researchers used ALS Beamline 5.3.1 and x-ray footprinting (XFP), a powerful technique in structural biology for the study of macromolecular structures and dynamics of proteins and nucleic acids in solution.
In any protein crystallography study, there is always the question of whether the crystallized protein represents the real-life protein, but with XFP you can look at proteins and/or nucleic acids in the solution state, and often under conditions that are close to physiological.
With the XFP study having confirmed the protein crystallography results as real, the next step for researchers will be to understand the detailed mechanism behind OCP’s interaction with the antenna to dissipate energy. Learning more about these might enable researchers to engineer smart photoprotection in cyanobacteria or even improve photosynthesis in green plants.
Contact: Cheryl Kerfield
Research conducted by: R. Leverenz (Michigan State University); M. Sutter (Michigan State University, Lawrence Berkeley National Laboratory); A. Wilson, A. Thurotte, D. Kirilovsky, and C. Bourcier de Carbon (Institut de Biologie et Technologies de Saclay); S. Gupta, C. Petzold, and C. Ralston (Lawrence Berkeley National Laboratory); F. Perreau (Institut Jean-Pierre Bourgin); and C.A. Kerfeld (Michigan State University, Lawrence Berkeley National Laboratory, UC Berkeley).
Research funding: U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES). Operation of the ALS is supported by DOE BES.
Publication about this research: R. Leverenz, M. Sutter, A. Wilson, S. Gupta, A. Thurotte, C. Bourcier de Carbon, C. Petzold, C. Ralston, F. Perreau, D. Kirilovsky, and C.A. Kerfeld, “A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection,” Science 348, 6242 (2015).
Adapted from the Berkeley Lab Science Short, “Orange is the New Red.”
ALS SCIENCE HIGHLIGHT #316