The Chemical Dynamics beamline was one of the first to be built at the Advanced Light Source (ALS), brought online just two years after the ALS achieved first light in 1993. It was a collaborative effort between the ALS and Berkeley Lab’s Chemical Sciences Division (CSD). Yuan T. Lee, a recipient of the 1986 Nobel Prize in Chemistry and, at the time, a senior faculty scientist in CSD, was a major early influence.
“Chemistry is the study of material transformations,” said Lee in his Nobel lecture. “Yet a knowledge of the rate, or time dependence, of chemical change is of critical importance for the successful synthesis of new materials and for the utilization of the energy generated by a reaction.”
Following this lead over the years and expanding on it, chemical dynamics researchers carved out a unique niche at the ALS, primarily using tunable vacuum ultraviolet (VUV) light to strip valence electrons from the atomic and molecular products of chemical reactions in progress, then identifying the photoionized products using mass spectrometry. Variations on this theme produced many highlights over time, on a wide range of topics, from combustion and environmental chemistry to astrochemistry and astrophysics.
“If you look at high-profile papers, there has been a lot of them for one little beamline like this,” said Musa Ahmed, a senior CSD scientist who’s been associated with the beamline in various roles for over 20 years. “I’ve stayed at the ALS all this time because I feel I’ve been able to help create a unique research environment that other synchrotrons actually try to emulate, and the ALS has allowed this to evolve.”
One important evolution was the creation of an umbrella group, led by Ahmed, that unified CSD programs at the ALS, including Molecular Environmental Science at Beamline 11.0.2. This structure encouraged greater interactions between CSD staff at the ALS and facilitated the free interchange of mobile endstations between beamlines—such “roll-up” endstations had been a hallmark of the chemical dynamics beamline from the beginning. The new organization also allowed the VUV beamline scientists to gain experience in using soft x-rays. “That kind of sowed the seeds of where we wanted to go with chemical dynamics,” said Ahmed.
There was room around the beamline for a second branchline, space that had previously been used for soft x-ray R&D using raw undulator light. Around 2014, then-director Roger Falcone proposed that the ALS could cost-effectively build a soft x-ray branch there by obtaining a monochromator from a recently decommissioned synchrotron facility at the University of Wisconsin. Usually costing $3–4 million, the monochromator was purchased for just $50,000, which turned out to be less than the cost of shipping it to Berkeley ($65,000).
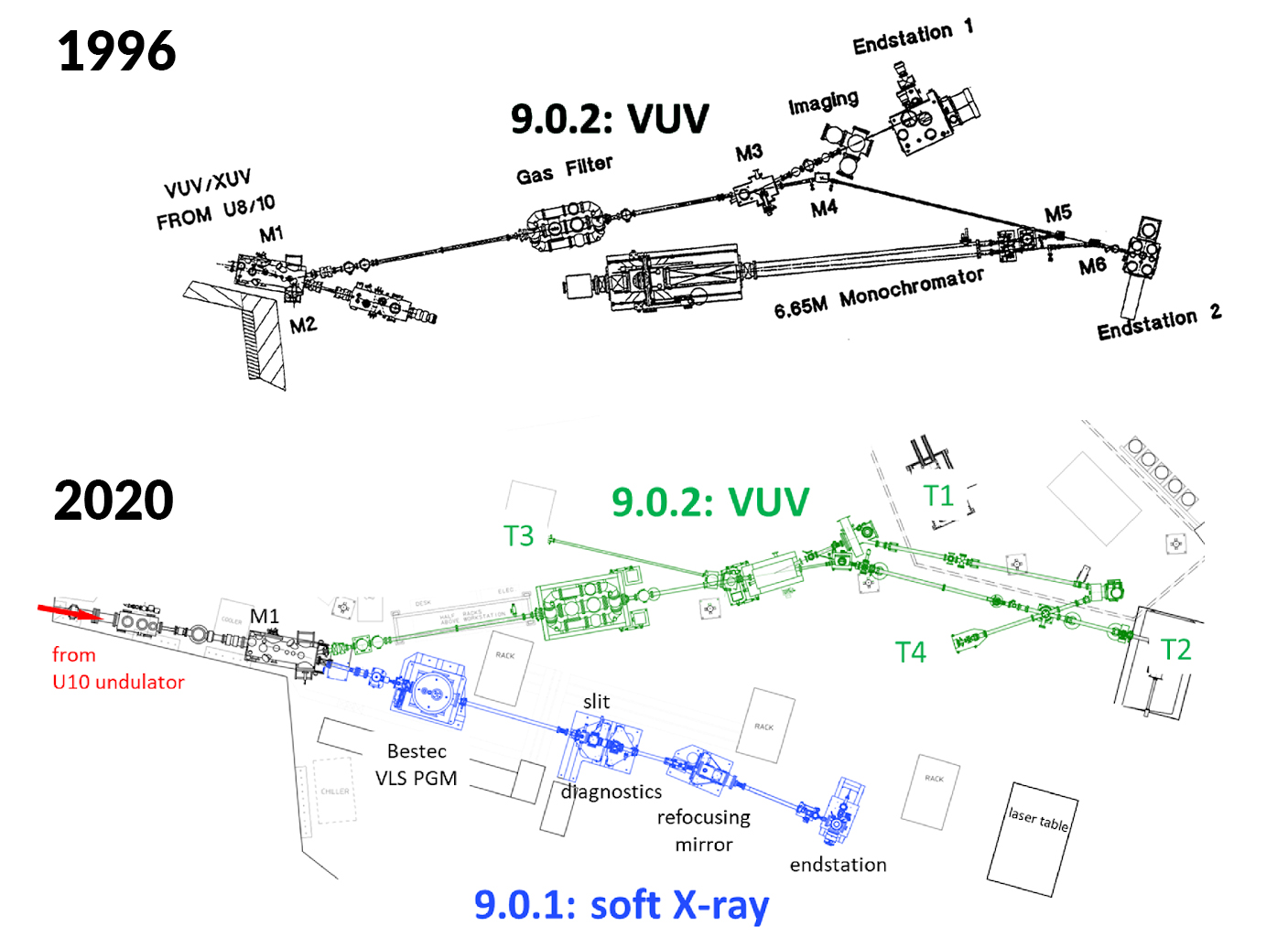
Anchored by the recycled monochromator, a new soft x-ray branch, 9.0.1, was built and commissioned by Oleg Kostko (ALS/CSD) and Bruce Rude (ALS), with help from Tony Warwick (ALS). It is now open to users on a limited basis, delivering soft x-rays with an energy range of 15 to 750 eV. This enables core-level photoelectron spectroscopy, complementing the valence-electron energies probed by the VUV branch, 9.0.2. A velocity-map imaging (VMI) detector—traditionally used to study gas-phase chemical dynamics—is used to record soft x-ray spectra of aerosols and unsupported nanoparticles, with full angular and energy distributions. Together, the soft x-ray and VUV branches comprise what’s now called the “Chemical Transformations” beamline, designated as Beamline 9.0.
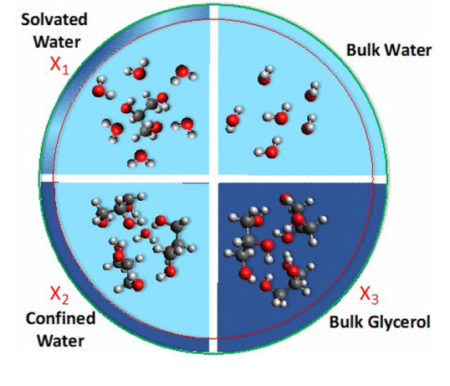
The first scientific paper to cite results from 9.0.1 was recently published in June (Weeraratna et al.). The researchers used a multimodal approach, coupling synchrotron-based x-ray photoelectron spectroscopy with (laser-based) terahertz and (tabletop) infrared spectroscopies to study hydrogen bonding in aerosols of glycerol and water. Glycerol—a viscous liquid at room temperature because of intra- and intermolecular hydrogen bonds—is used as a vaporizing solvent in e-cigarettes to produce aerosols that simulate tobacco smoke.
Studies such as this provide insights into evaporation, diffusion dynamics, and reactivity in aqueous aerosols, which are not only relevant to understanding the effects of tobacco smoke and vaping, but also to atmospheric chemistry and the spread of illnesses such as COVID-19 via respiratory droplets and aerosols.
“Our forte has always been to get aerosol jets and molecules into the gas phase,” said Ahmed. “We’re still using the same methods, but now we’re trying to tackle that complexity in clusters, aerosols, and nanoparticles using both VUV and soft x-ray radiation.”
In the future, Ahmed envisions the development of capabilities for performing simultaneous spectroscopy and scattering experiments, in order to incorporate structural information about reactive systems in real time. Pump-probe experiments can still benefit from the MHz repetition rates possible at synchrotron sources. And, with the planned ALS Upgrade Project (ALS-U) on the horizon, powerful emerging x-ray methods such as x-ray photon correlation spectroscopy (XPCS) and resonant inelastic x-ray scattering (RIXS), heroic at third-generation light sources, should become routine, including at the Chemical Transformations beamline.
“The way I look at it, we’ve come a long way in the past 25 years, and this is how we are going to do chemistry for the next 25 years,” said Ahmed. “Multimodal, soft x-rays, tunable lasers coupled to the synchrotron, you know, all that good stuff.”
Read more: M. Ahmed and O. Kostko, “From atoms to aerosols: probing clusters and nanoparticles with synchrotron based mass spectrometry and X-ray spectroscopy,” Phys. Chem. Chem. Phys. 22, 2713 (2020).