The promise of cost-effective devices that cleanly convert fuel into electricity (or vice versa) is limited by the inefficiency and instability of cheaper alternatives to platinum (Pt), a well-studied catalyst for the electrochemical reactions involved. To understand how to make better-performing alternatives, we need a closer look at the interface between two key components in such devices—the Pt electrode and the liquid electrolyte—under realistic (“operando”) conditions. What is really happening when water is being split to form oxygen (the “oxygen evolution reaction”) at the platinum electrode in alkaline conditions?
Using ambient-pressure x-ray photoelectron spectroscopy (APXPS) with “tender” x-rays (4 keV) at ALS Beamline 9.3.1, researchers studied the solid/liquid interface of Pt samples in contact with an alkaline electrolyte in a fully operating electrochemical cell under a variable applied voltage. Ex situ atomic-force microscopy was also used to probe the platinum surface morphology after various applied potentials.
The results revealed the molecular-level chemistry, structure, and dynamics of the Pt surface, highlighting differences between thermodynamic predictions and the actual surface composition under realistic working conditions. The researchers were able to combine and reconcile various proposed mechanisms into a more complete picture of how oxygen evolves at a Pt interface undergoing the water-splitting reaction in alkaline conditions. They found that the rate-determining step does not occur in the outermost layer, but in a porous buried volume consisting of “electronically activated” Pt sites (Ptδ) that selectively bind OH−. The work demonstrates a new experimental capability that will deepen our knowledge and accelerate the development of novel tailored materials for the clean generation of energy.
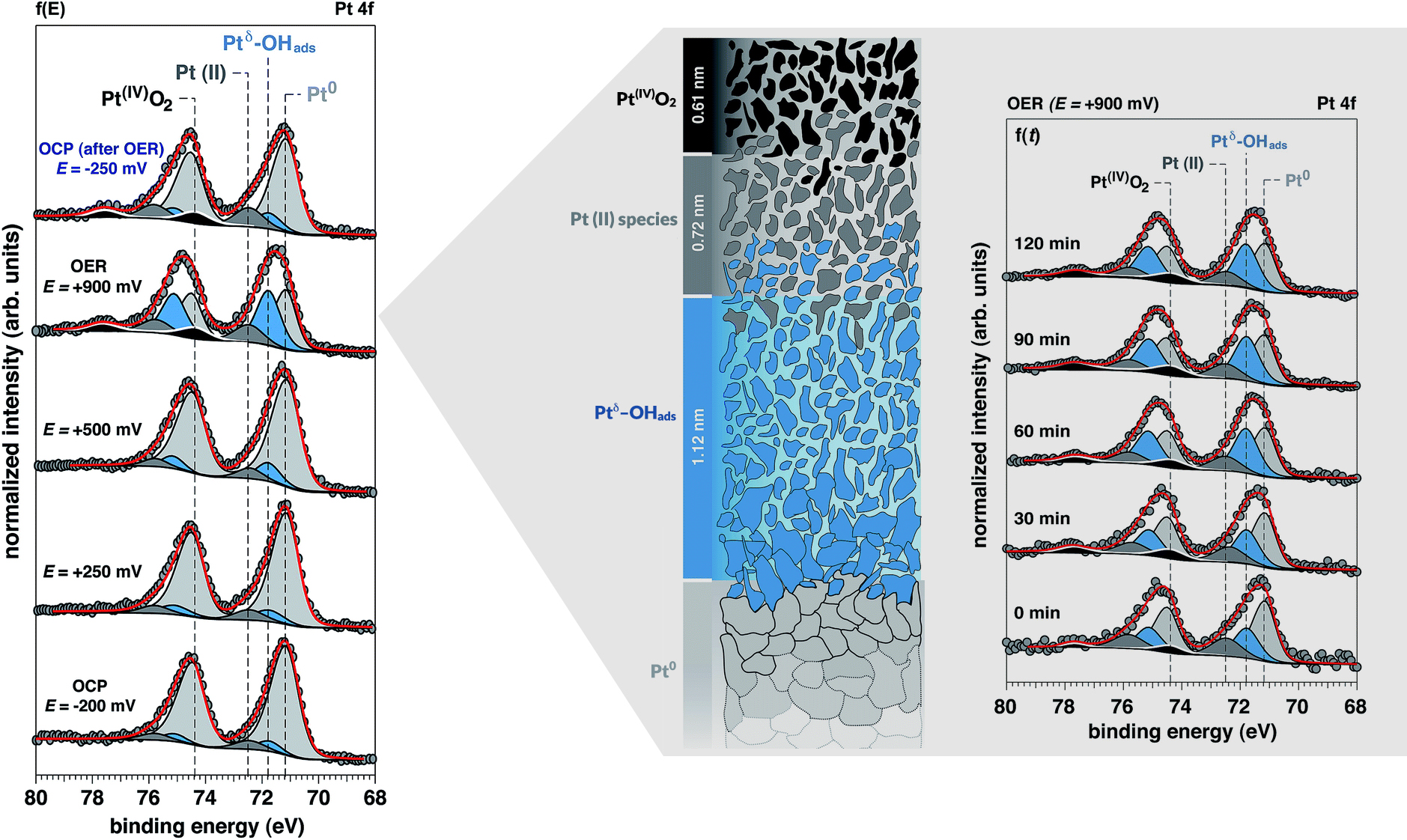
Work performed at ALS Beamline 9.3.1.
M. Favaro, C. Valero-Vidal, J. Eichhorn, F.M. Toma, P.N. Ross, J. Yano, Z. Liu, and E.J. Crumlin, “Elucidating the alkaline oxygen evolution reaction mechanism on platinum,” J. Mater. Chem. A (2017), doi: 10.1039/C7TA00409E.