Sulfur dioxide (SO2) and nitrogen dioxide (NO2) emissions from fossil-fuel combustion are harmful to the environment and to human health. Current technologies for reducing such emissions have several disadvantages, including the incomplete removal of pollutants and the generation of large volumes of waste.
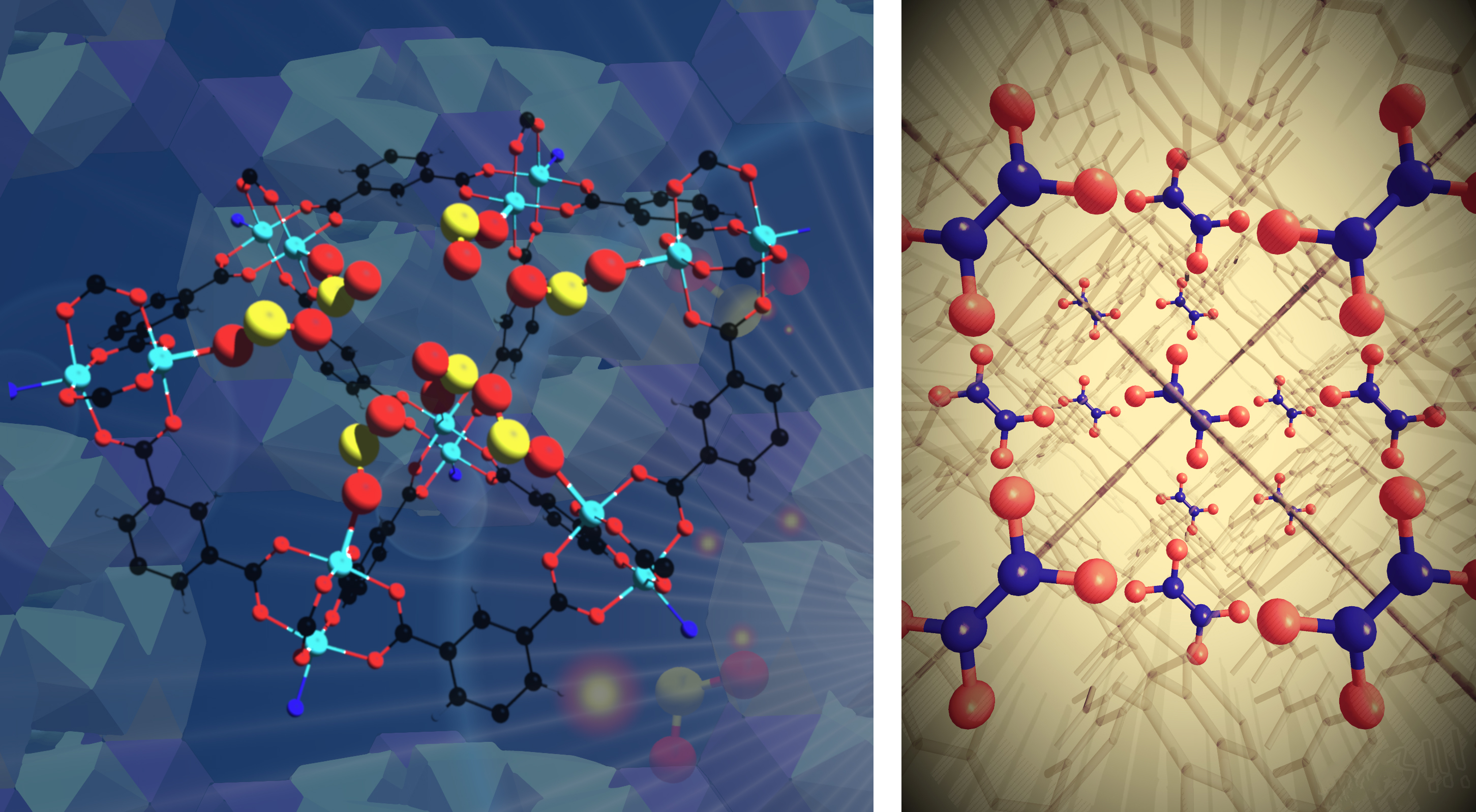
Researchers are therefore seeking new approaches to exhaust-gas clean-up based on metal–organic frameworks (MOFs): cage-like structures that can capture and store molecules. The choice of metal ions and the size, shape, and chemical properties of the organic linkers can be changed to tune the material for a desired application. In the two studies described here, researchers synthesized two MOFs (MFM-170 and MFM-520) capable of selectively capturing even trace quantities of SO2 and NO2, respectively. After saturation, both materials can be regenerated and reused many times, reducing the problem of waste.
At ALS Beamline 11.3.1, x-ray diffraction experiments were performed on gas-loaded single crystals to determine the precise location and mechanism of SO2 and NO2 binding within the materials. For MFM-170, this was the first crystallographically observed example of SO2 coordination to a MOF metal site, a process that was found to be fully reversible. In MFM-520, the researchers confirmed the dimerization of NO2 to N2O4, a precursor of the commodity chemical, nitric acid. Further analyses were conducted at Diamond Light Source and ISIS Neutron and Muon Source in the UK, and at Oak Ridge National Laboratory.
In sum, the examined materials have the potential to be used for many capture-and-release cycles without the production of waste, and most crucially, can permit the conversion of SO2 and NO2 into valuable chemical products.
G.L. Smith, J.E. Eyley, X. Han, X. Zhang, J. Li, N.M. Jacques, H.G.W. Godfrey, S.P. Argent, L.J. McCormick McPherson, S.J. Teat, Y. Cheng, M.D. Frogley, G. Cinque, S.J. Day, C.C. Tang, T.L. Easun, S. Rudić, A.J. Ramirez-Cuesta, S. Yang, and M. Schröder, “Reversible coordinative binding and separation of sulfur dioxide in a robust metal–organic framework with open copper sites,” Nat. Mater. 18, 1358 (2019), doi:10.1038/s41563-019-0495-0; and J. Li, X. Han, X. Zhang, A.M. Sheveleva, Y. Cheng, F. Tuna, E.J.L. Mcinnes, L.J. McCormick, S.J. Teat, L.L. Daeman, A.J. Ramirez-Cuesta, M. Schröder, and Sihai Yang, “Capture of nitrogen dioxide and conversion to nitric acid in a porous metal-organic framework,” Nat. Chem. 11, 1085 (2019), doi: 10.1038/s41557-019-0356-0.