Researchers have found novel nanocatalysts that lower the barrier to converting carbon dioxide (CO2)—an abundant greenhouse gas—into methanol (CH3OH)—a key commodity used to produce numerous industrial chemicals and fuels. With the help of ambient-pressure x-ray photoelectron spectroscopy (AP-XPS) at the ALS, researchers have discovered that nanoparticles of cerium oxide (ceria) in contact with copper will form metal–oxide interfaces that allow the adsorption and activation of CO2, opening a new reaction pathway for the synthesis of methanol. In one case, it worked almost 90 times faster than catalysts commonly used for this reaction today.
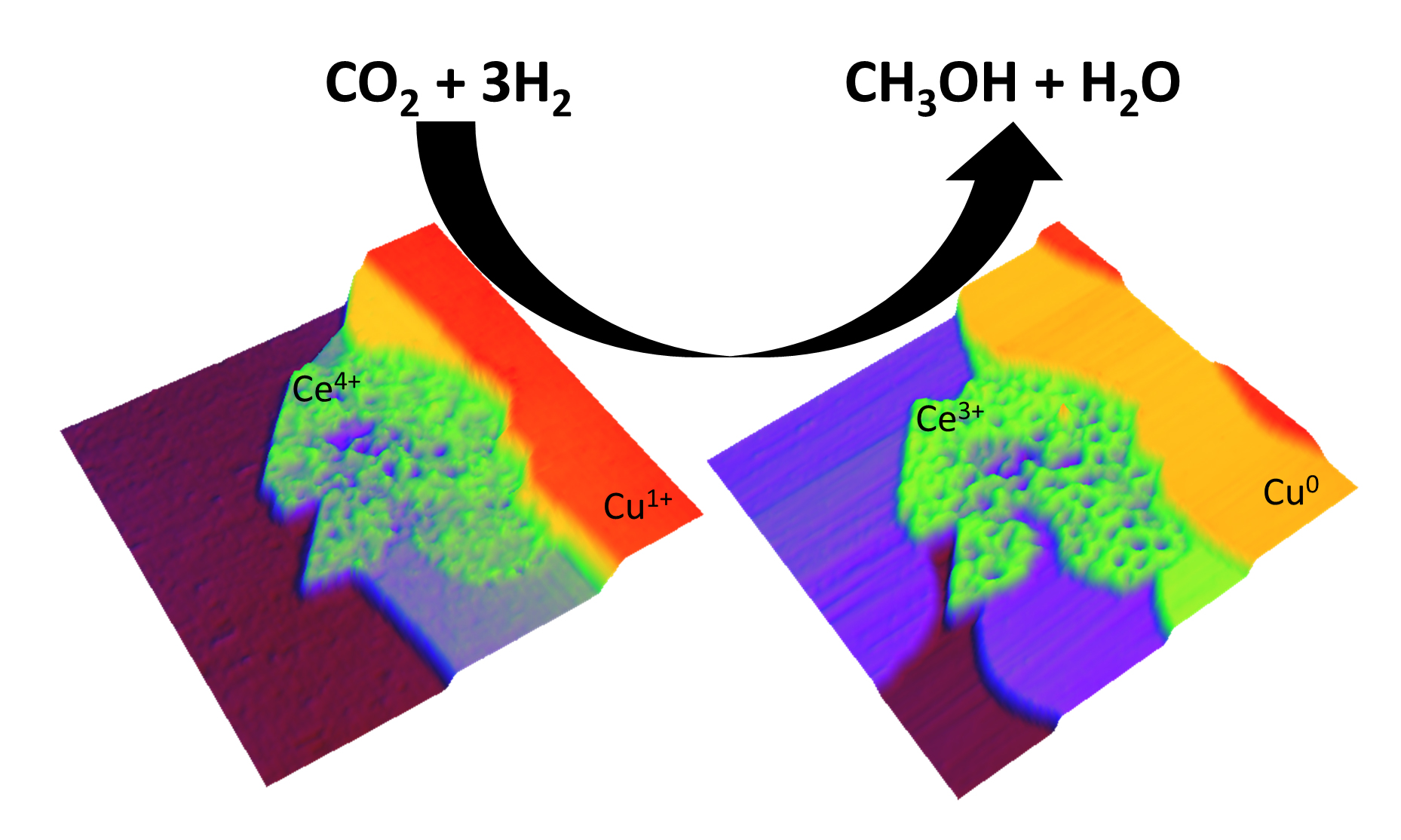
Currently, methanol is synthesized industrially from mixtures of H2 and CO (or sometimes CO2) at elevated pressures (50 to 100 atm) and temperatures (450 to 600 K) using catalysts containing copper and zinc oxide. The synthesis of methanol from CO2 is of particular interest as a way to mitigate this abundant greenhouse gas and make use of it as an alternative and economical methanol feedstock. However, because of its chemical inertness, CO2 interacts only weakly with most catalysts. Previous studies have shown that its reactivity can be enhanced somewhat by “decorating” a Cu surface with Zn or late transition metals. In this work, the researchers took a different approach, coupling Cu to ceria (CeOx), a reducible oxide. The ceria/Cu system had been studied before as a catalyst for other reactions. The cations of the ceria nanoparticles in contact with Cu can easily alternate between 3+ and 4+ oxidation states. Thus, in ceria/Cu, an active metal–oxide interface can have oxide centers with dynamic chemical properties.
In this study, about 20% of the catalyst’s Cu substrate was covered by ceria. The nanostructure was prepared by vapor-depositing Ce onto Cu in an atmosphere of O2 to form ceria islands on the step edges of a Cu surface. The ceria islands were predominantly one layer thick, exhibiting rough surfaces with an occasional CeO2 termination. Reduction in H2 increased the surface roughness of the ceria particles, creating an expanded ceria–Cu interface.
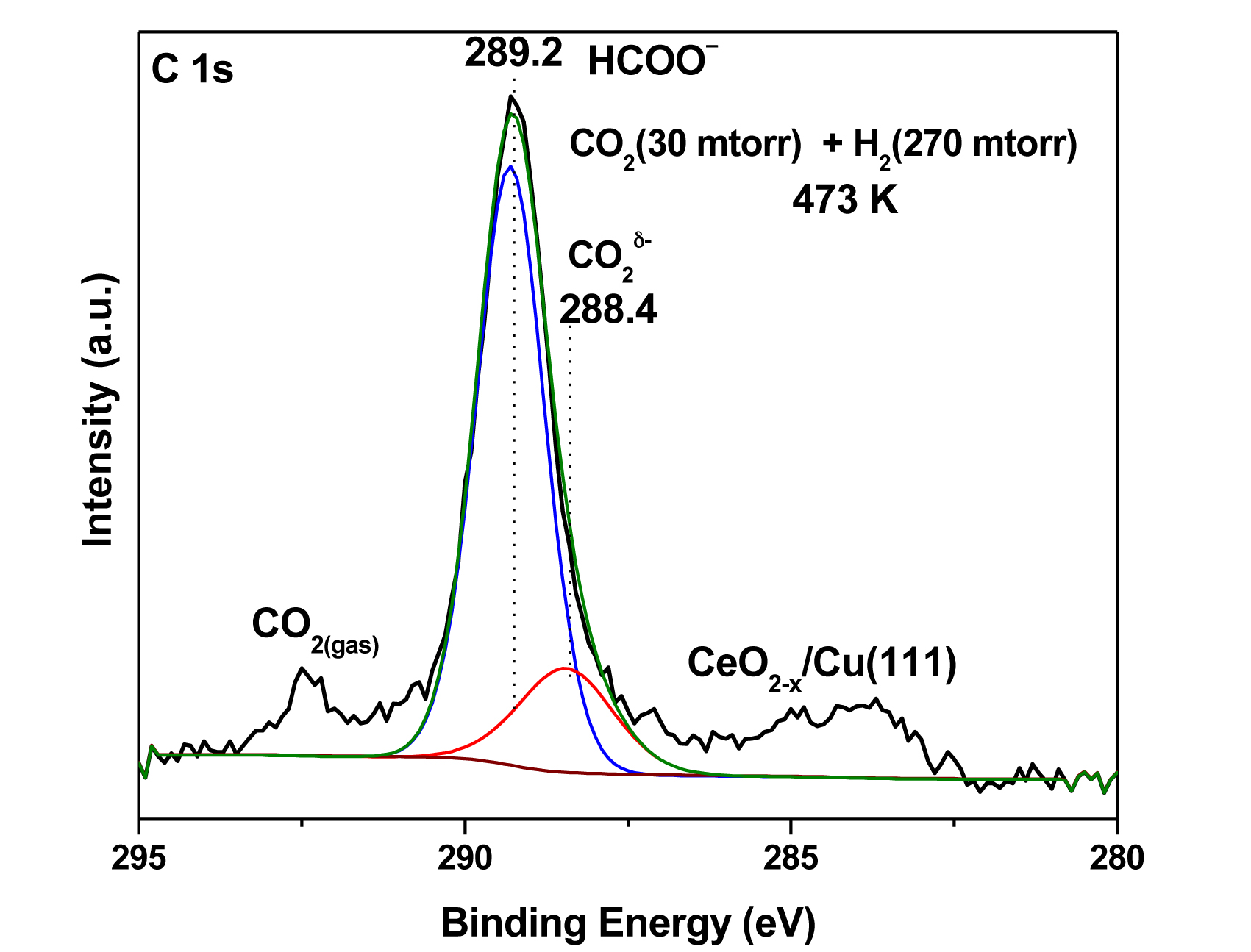
Using a combination of AP-XPS at ALS Beamline 9.3.2 and infrared spectroscopy, the researchers investigated the interaction of CO2 and CO2/H2 gas mixtures with Cu, ceria, and ceria/Cu surfaces at temperatures between 300 and 500 K. Pure CO2 did not adsorb on Cu at these temperatures. On the other hand, the adsorption of CO2 on a ceria surface produced strongly bound carbonate (CO32-) species. A carboxylate (CO2δ-) species was detected in appreciable amounts only when ceria–copper interfaces were present in the catalyst. The low stability of the carboxylate species makes it an excellent intermediate in the conversion of CO2 to methanol.
The results of DFT calculations indicated that the thermochemistry of the reaction steps associated with the formation of methanol on a ceria–copper interface is predominantly downhill, with an overall exothermic process.
The researchers also investigated a catalyst generated by co-depositing nanoparticles of Cu and ceria on a substrate of titania (TiO2). The rate of methanol production for this catalyst was about 87 times faster than for the typical catalyst of copper and zinc oxide in use today, while plain ceria on titania (without Cu) showed no activity for methanol synthesis. The results suggest that the extremely high activity is probably attributable to the type of metal–oxide interface involving Cu and ceria described above.
In general, this study illustrates the substantial benefits that can be obtained by properly tuning the properties of a metal–oxide interface in catalysts for methanol synthesis. In a metal–oxide interface, one can have adsorption and reaction sites with complementary chemical properties, truly bifunctional sites that would be very difficult to generate on the surface of pure metal or alloy systems.

Contact: Jose Rodriguez
Research conducted by: J. Graciani and J. Fernández Sanz (Univ. of Seville, Spain); J. Evans (Univ. Central de Venezuela); and K. Mudiyanselage, F. Xu, A.E. Baber, S.D. Senanayake, D.J. Stacchiola, P. Liu, J. Hrbek, and J.A. Rodriguez (Brookhaven National Laboratory).
Research funding: Venezuelan Institute of Petroleum Technology (Intevep), Spanish Ministry of Economy and Finance, European Regional Development Fund, and U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES). Operation of the ALS is supported by DOE BES.
Publication about this research: J. Graciani, K. Mudiyanselage, F. Xu, A.E. Baber, J. Evans, S.D. Senanayake, D.J. Stacchiola, P. Liu, J. Hrbek, J. Fernández Sanz, and J.A. Rodriguez, “Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2,” Science 345, 546 (2014). doi:10.1126/science.1253057
ALS SCIENCE HIGHLIGHT #302