SCIENTIFIC ACHIEVEMENT
Researchers used crystallography at the Advanced Light Source (ALS) to trace the step-by-step path of water-molecule uptake in a porous compound, then made pinpoint modifications to shape the material’s water-sorption behavior.
SIGNIFICANCE AND IMPACT
The results led to improvements in the compound’s efficiency at harvesting water from the air, an important step toward alleviating water shortages in the future.
Pulling water out of thin air
While moisture farms in the desert remain in the realm of science fiction for now, the ability to extract water from dry air is already science fact. This is very good news, as increasing water use and changes in climate patterns have made water scarcity a growing global concern, affecting billions of people. “The atmosphere has almost as much water at any one time as all the rivers and lakes,” said Omar Yaghi, a professor of chemistry at UC Berkeley. “Harvesting this water could help turn dry deserts into oases.”
Yaghi’s group has been developing porous materials, known as metal–organic frameworks (MOFs), that enable the extraction of moisture from the air. MOFs are scaffold-like molecules constructed of inorganic metal clusters connected to each other by organic linker molecules. This framework leaves ample room for “guest” water molecules to move in and out of its pores.
In previous work, the group successfully tested aluminum-based MOF-303 in the Mojave Desert, capturing about three cups of water per kilogram of absorber per day. To design MOFs that are even more efficient, productive, and affordable, the researchers needed a deeper understanding of water–framework interactions.
Adsorption mechanism, step by step
Although the positions of water molecules in porous crystals have been identified in the past, determining the water-filling sequence has been very challenging. In this study, the team, in collaboration with Laura Gagliardi (University of Chicago) and Joachim Sauer (Humboldt University of Berlin), used single-crystal x-ray diffraction at ALS Beamline 12.2.1 to decipher the water-uptake mechanism of MOF-303.
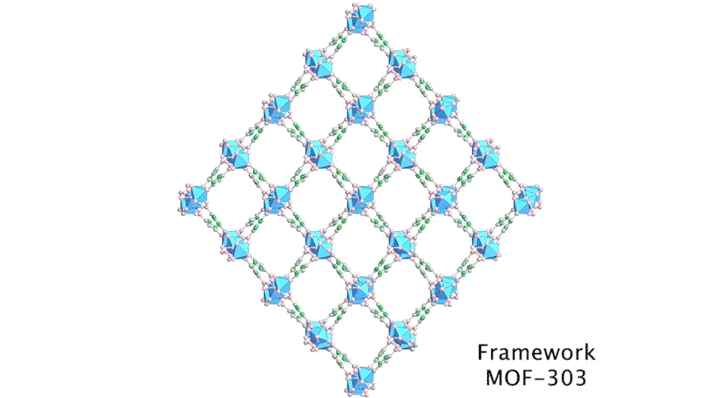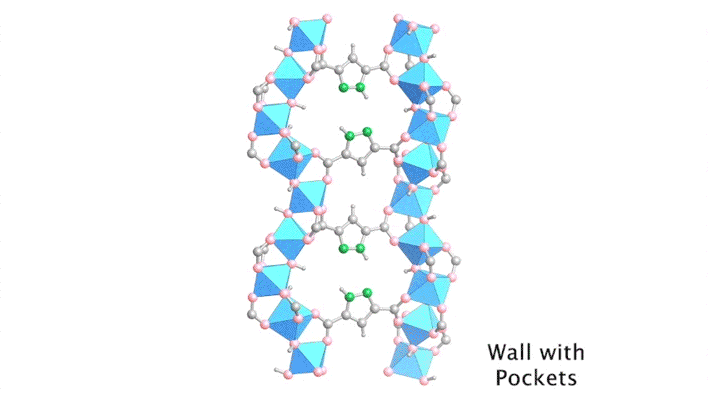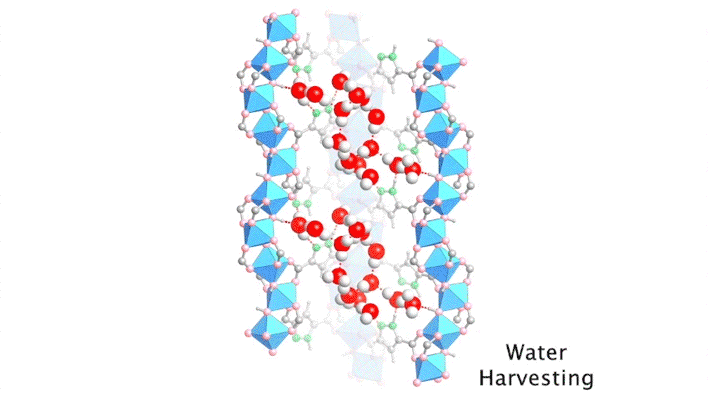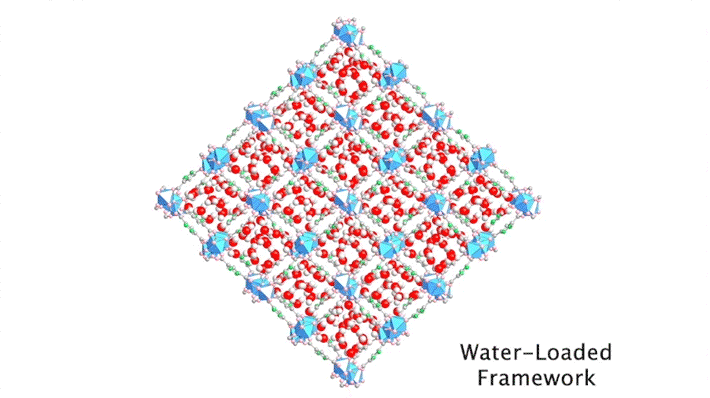
The high intensity of the synchrotron allowed the researchers to capture a series of snapshots of the water structures at different water loadings within a short period of time, a feat that would not have been possible using a conventional diffractometer. By gradually ramping up the temperature of a dry protective gas stream, it was possible to closely monitor the slow, controlled desorption of the water molecules, one by one.
By sequencing the individual snapshots into a movie, the researchers were able to clearly see how the framework contracts and expands during the loading process. More crucially, they were able to see how the first water molecules bind to the strongest adsorption sites; these were followed by additional water molecules forming isolated clusters, then chains of clusters, and finally a water network.
Swapping linkers for greater efficiency
With the help of the ALS data, the researchers identified the strongest adsorption sites in the MOF—i.e., the sites that hold most tightly to the water molecules. With this knowledge, they were able to swap out certain organic linkers to modulate the water–framework interactions, reduce the desorption (water-release) temperature, and make the water-harvesting process more efficient.
In the future, the team would like to apply this characterization approach to a broader range of MOFs (or materials in general). With the understanding gained through such studies, it would then be possible to develop on-demand materials for atmospheric water capture, rather than synthesize new sorbents through trial and error. In the long term, the researchers envision that this technology will facilitate water harvesting from air and make this technology more affordable to the people who need it the most.
This video from UC Berkeley, featuring chemistry professor Omar Yaghi and members of his group, chronicles a successful field test of a prototype water harvester a few years ago. The water harvester was able to extract drinkable water every day/night cycle at very low humidity and at low cost, making it ideal for people living in arid, water-starved areas of the world. (Video credit: Roxanne Makasdjian and Stephen McNally/UC Berkeley Public Affairs)
Contacts: Nikita Hanikel and Omar Yaghi
Researchers: N. Hanikel, X. Pei, H. Lyu, and O.M. Yaghi (University of California, Berkeley); S. Chheda and W.S. Jeong (University of Minnesota); J. Sauer (Humboldt University of Berlin, Germany); and L. Gagliardi (University of Chicago).
Funding: Defense Advanced Research Projects Agency, National Institutes of Health, German Academic Scholarship Foundation, Kavli Energy NanoSciences Institute (ENSI), and German Research Foundation. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: N. Hanikel, X. Pei, S. Chheda, H. Lyu, W.S. Jeong, J. Sauer, L. Gagliardi, and O.M. Yaghi, “Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting,” Science 374, 454 (2021), doi:10.1126/science.abj0890.
ALS SCIENCE HIGHLIGHT #459