SCIENTIFIC ACHIEVEMENT
At the Advanced Light Source (ALS), researchers developed a new infrared approach to probing the first few molecular layers of a liquid in contact with a graphene electrode under operating conditions.
SIGNIFICANCE AND IMPACT
The work offers a new way to study the interfaces that are key to understanding batteries, corrosion, and other bio- and electrochemical phenomena.
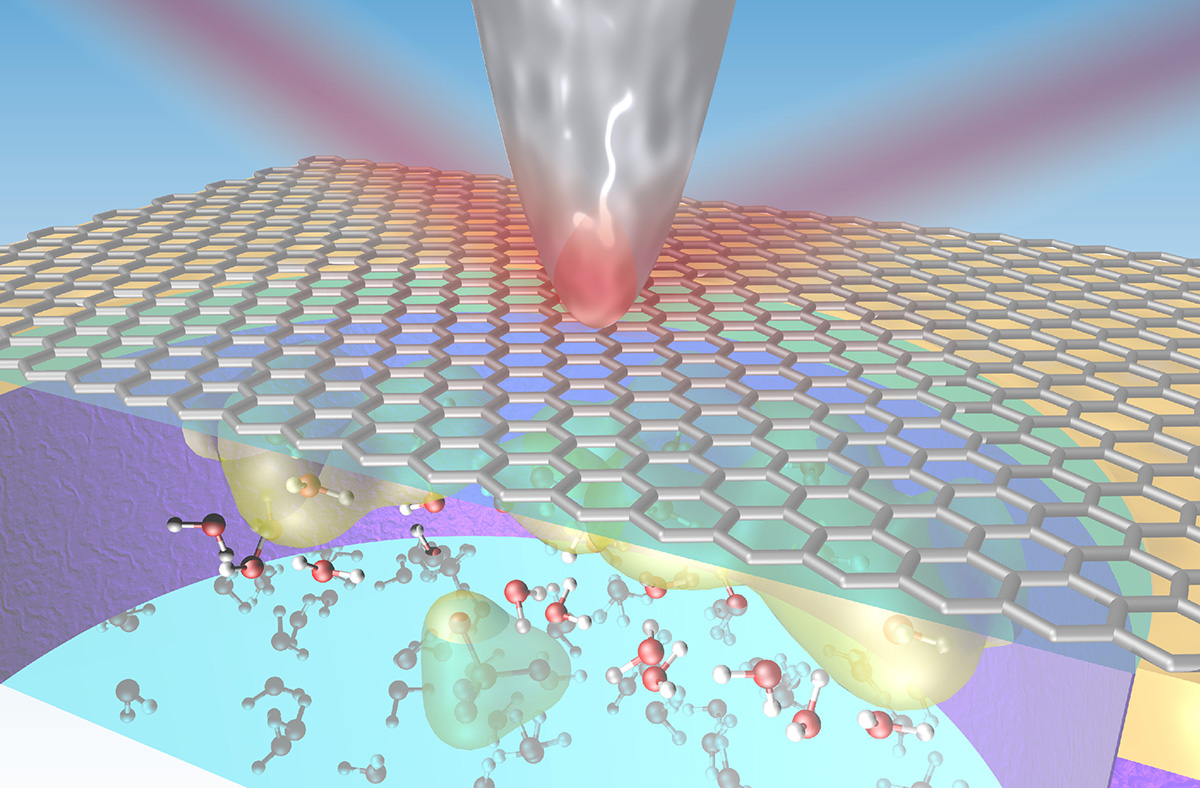
Interface investigations: deep undercover
Solid–liquid interfaces are vital to physical and chemical processes in many subjects, including corrosion, the generation and storage of energy (fuel cells, batteries), biology (ion transport through cell membranes), and environmental science (chemical weathering). However, solid–liquid interfaces are difficult to experiment on because they are buried under the bulk of the materials on either side of the interface. Furthermore, the interface region is extremely thin—only a few atomic layers. Many techniques for probing materials reveal just surface information, or, if they can penetrate more deeply, provide information averaged over a large volume, rather than from a thin slice close to an interface. Now, researchers have demonstrated a way to overcome these challenges, using synchrotron infrared nanospectroscopy (SINS) at the ALS.
Double-checking the double layer
Numerous theories have been developed to describe the structure of solid–liquid interfaces, including the electrical double layer (EDL) concept first proposed by Hermann von Helmholtz in the 1800s. In this model, a charged solid surface (an electrode) exposed to a liquid with free-floating ions (an electrolyte solution) will attract a layer of oppositely charged ions. A second layer of ions, more diffuse and loosely bound, extends into the electrolyte. Despite its importance to basic phenomena such as charge transfer, adsorption, and capacitance, the EDL model requires further experimental validation at the molecular level, for a deeper understanding of interface chemistry that can be applied toward the improvement of many technologies.
SINS in a graphene-capped liquid cell
In this work, researchers from several Berkeley Lab divisions—Materials Sciences, Energy Storage and Distributed Resources, the Molecular Foundry, and the ALS—developed a way to collect infrared vibrational spectra at a graphene–electrolyte interface with nanoscale spatial resolution. In SINS experiments at ALS Beamlines 2.4 and 5.4, the light scattered from the interaction between the tip of an atomic force microscope probe and the sample is analyzed using Fourier transform infrared nanospectroscopy (nano-FTIR).
A key component of the technique is a liquid cell with a reservoir of several microliters, capped by a graphene membrane. The graphene is transparent to photons, impermeable to gases and liquids, and acts as a working electrode for studies performed under an applied voltage. With this arrangement, the researchers performed infrared spectroscopy studies with water, propylene carbonate, and aqueous ammonium sulfate electrolyte solutions.

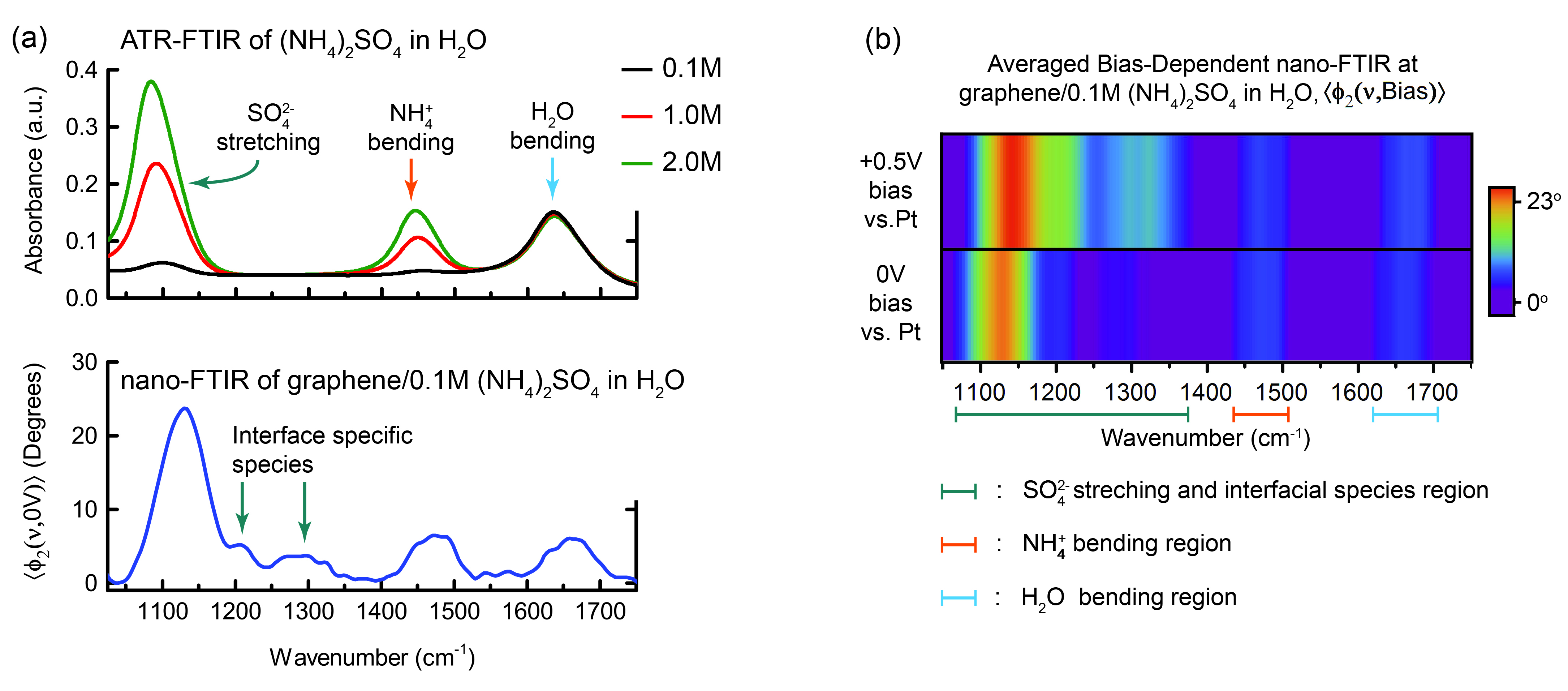
Comparison with conventional FTIR
The nano-FTIR spectra of water and propylene carbonate matched those measured using conventional FTIR (ATR-FTIR), indicating the success of the liquid-cell platform. More importantly, the power of the method was demonstrated by the results on ammonium sulfate, which contains ions identifiable by their vibrational characteristics. Several additional peaks appeared in the nano-FTIR spectrum, and the relative peak intensities differed from the ATR-FTIR spectra. In measurements under an applied voltage, a clear bias-dependent feature appeared.
These results demonstrate the ability to obtain spectroscopic data from within the EDL and diffuse layer not seen using conventional FTIR, opening the door to future simulation work identifying the composition of these layers. In the longer term, the approach paves the way for nondestructive, in situ, and operando investigations of liquid environments and solid–liquid interfaces, particularly for applications in biology, energy storage, and electrochemistry.

Contacts: Yi-Hsien Lu and Jonathan Larson
Researchers: Y.-H. Lu, J.M. Larson, A. Baskin, P.D. Ashby, D. Prendergast and R. Kostecki (Berkeley Lab); X. Zhao and M. Salmeron (Berkeley Lab and UC Berkeley); and H.A. Bechtel (ALS).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES); and Energy Biosciences Institute (EBI)-Shell Research Program. Operation of the ALS is supported by DOE BES.
Publication: Y.-H. Lu, J.M. Larson, A. Baskin, X. Zhao, P.D. Ashby, D. Prendergast, H.A. Bechtel, R. Kostecki, and M. Salmeron, “Infrared Nanospectroscopy at the Graphene–Electrolyte Interface,” Nano Lett. 19, 5388 (2019), doi:10.1021/acs.nanolett.9b01897.
ALS SCIENCE HIGHLIGHT #406