SCIENTIFIC ACHIEVEMENT
In lithium batteries after fast charging, researchers measured the persistence of internal currents and found that large local currents continue even after charging has stopped.
SIGNIFICANCE AND IMPACT
The work uses hard x-ray 3D imaging at the Advanced Light Source (ALS) in a novel way and sheds light on the causes of thermal runaway and the catastrophic failure of lithium batteries at rest.
The mystery of thermal runaway at rest
How likely is an electric-vehicle (EV) battery to self-combust and explode? The chances of that happening are actually pretty slim: Some analysts say that gasoline vehicles are nearly 30 times more likely to catch fire than EVs. But recent news of EVs catching fire while parked have left many consumers—and researchers—scratching their heads over how these rare events could possibly happen.
Researchers have long known that high electric currents can lead to “thermal runaway”—a chain reaction that can cause a battery to overheat, catch fire, and explode. But without a reliable method to measure currents inside a resting battery, it has not been clear why some batteries go into thermal runaway when an EV is parked.
Now, by using operando x-ray microtomography at the ALS, scientists have shown that the presence of large local currents inside batteries at rest after fast charging could be one of the causes behind thermal runaway.
A source of local ionic current
In a lithium-ion battery, the anode is mostly made of graphite. When a healthy battery is charged slowly, lithium ions from the cathode weave themselves between the layers in graphite particles, which can then reach a full state of charge. In contrast, when the battery is charged rapidly, the lithium ions have a tendency to plate on the surface of the graphite in the form of lithium metal.
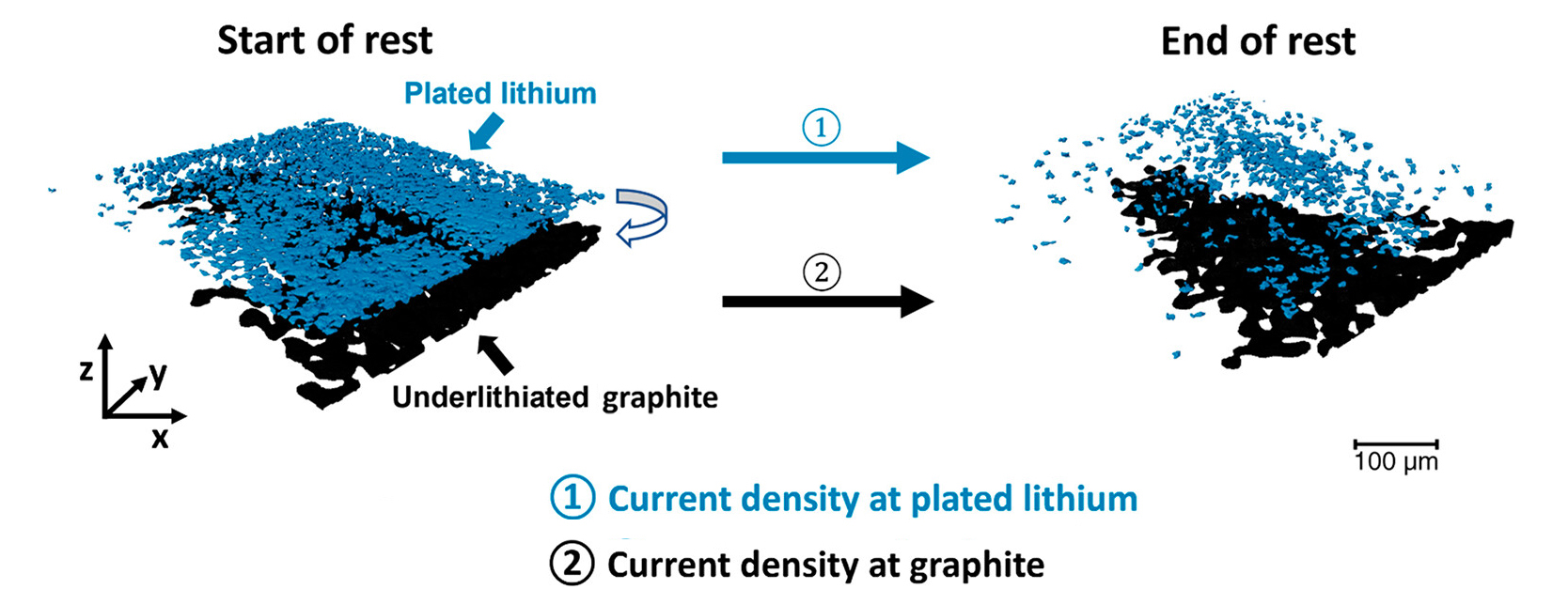
Previous microtomography experiments at the ALS indicated that these deposits cast lithiation “shadows”—regions of poor lithiation at the back of the graphite electrode. When this happens, even though the battery is at rest, there is a potential difference between the plated lithium metal and the underlithiated particles that can drive local ionic currents. Measuring these time-dependent currents requires a nondestructive operando technique that’s also spatially resolved, because the lithium plating is not uniform across the electrode.
Peering inside a battery’s undercurrents
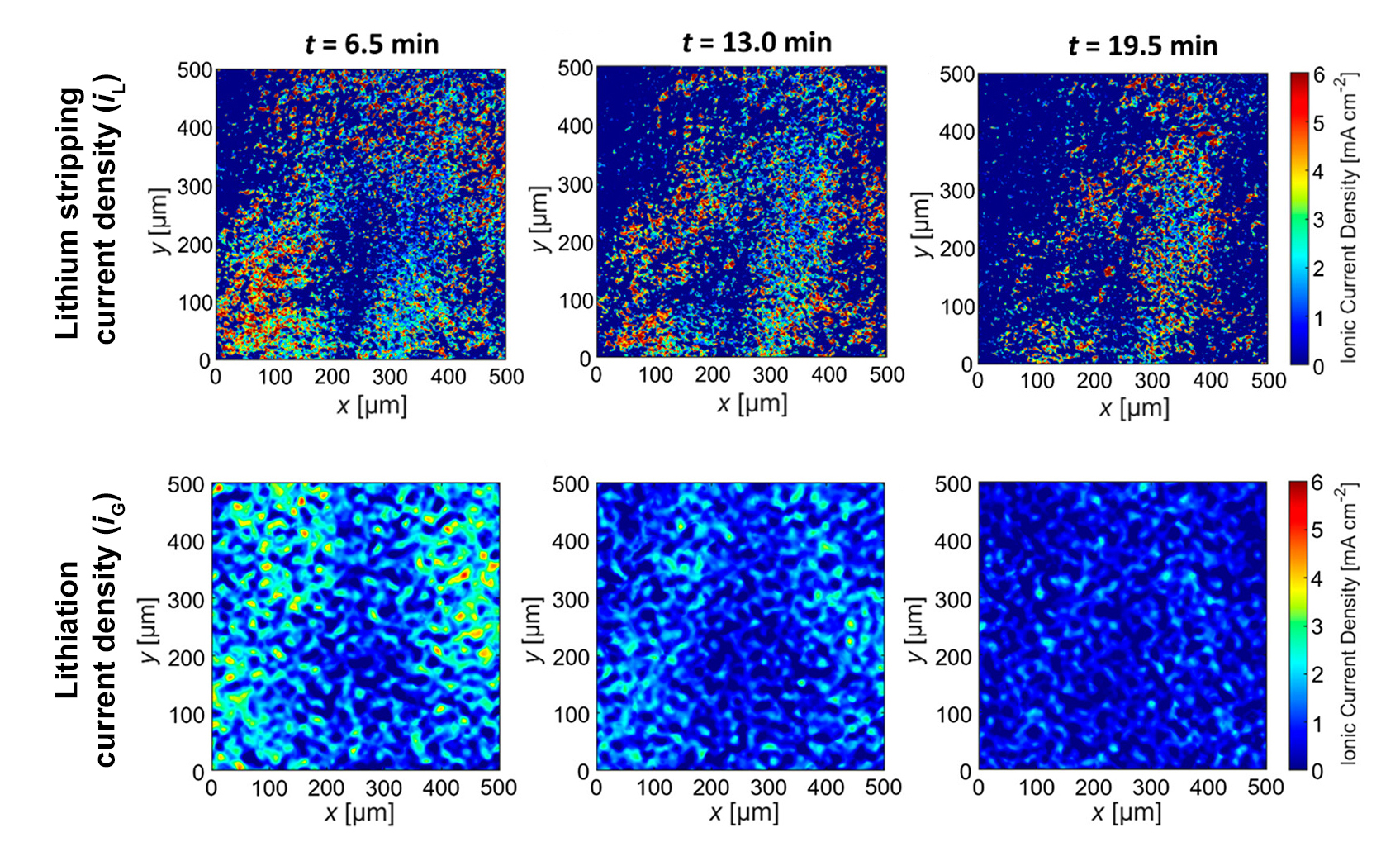
At ALS Beamline 8.3.2, the researchers were able to tomographically measure slight expansions in the graphite particle sizes as lithiation increased. This provided them with information about the degree of lithiation in the graphite at specific times after fast charging. They also measured changes in the volume of the plated lithium layer at the same time intervals. With this data, the researchers were able to determine the spatially resolved ionic current densities in the lithium and graphite (iL and iG, respectively).
The results revealed that, after charging the battery in 10 minutes, the average current densities decreased from 1.5 to 0.5 mA/cm2 in about 20 min after charging stopped. Surprisingly, however, the range of the lithium current density was independent of time, with outliers generating alarming current densities as high as 25 mA/cm2. In comparison, the current density required to charge the battery in 10 minutes was 18 mA/cm2.
The persistence of these outliers provides a clue as to the origin of catastrophic failure in batteries at rest. However, much more work is needed before this approach can be used to develop improved safety protocols.
Contact: Nitash Balsara
Researchers: A.S. Ho and N.P. Balsara (UC Berkeley and Berkeley Lab), D.Y. Parkinson (ALS), and S.E. Trask and A.N. Jansen (Argonne National Laboratory).
Funding: US Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Techologies Office; and the National Science Foundation. Operation of the ALS is supported by the US DOE, Office of Science, Basic Energy Sciences program.
Publication: A.S. Ho, D.Y. Parkinson, S.E. Trask, A.N. Jansen, and N.P. Balsara, “Large Local Currents in a Lithium-Ion Battery during Rest after Fast Charging,” ACS Nano 17, 19180 (2023), doi:10.1021/acsnano.3c05470.
Adapted from the Berkeley Lab news release, “Why Do Batteries Sometimes Catch Fire and Explode?”
ALS SCIENCE HIGHLIGHT #494