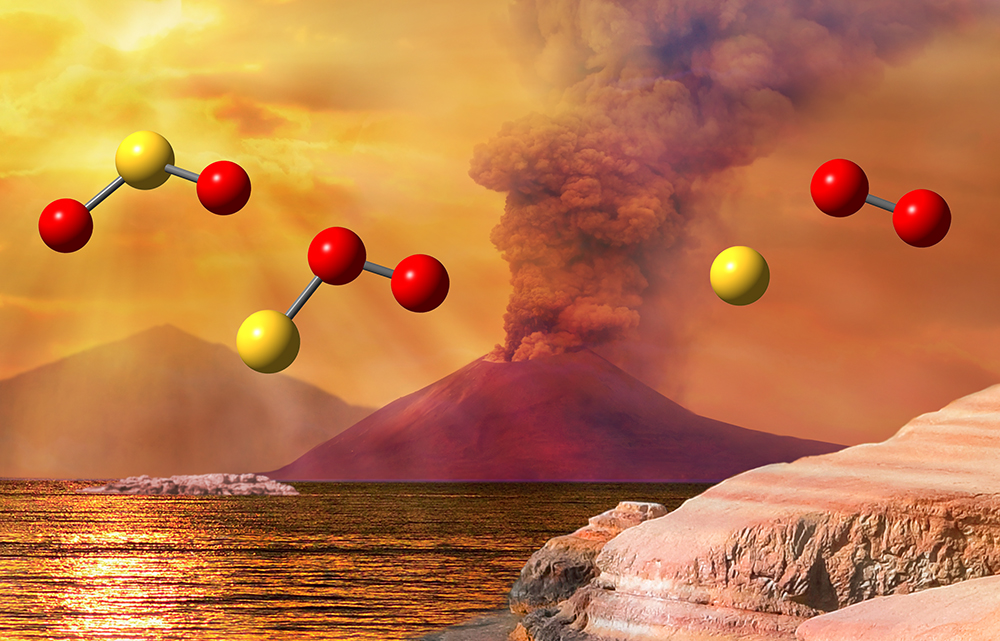
While calibrating a new scientific apparatus at the Advanced Light Source (ALS), researchers discovered that ultraviolet light can break up sulfur dioxide (SO2) in a new way, with molecular oxygen (O2) as an unexpected product. The discovery sheds light on Earth’s Great Oxygenation Event 2.4 billion years ago, when atmospheric oxygen levels first began to rise.
The researchers, from Sandia National Laboratories in Livermore, California, were testing a new mass spectrometry instrument at the ALS’s Chemical Dynamics beamline (Beamline 9.0.2). They were using it to break a chemical bond in SO2 with ultraviolet light at a wavelength of 193 nm, which has been well documented to produce only SO and O. Surprisingly, the researchers also saw a small signal from S and O2.
“At first we thought this was an error,” said David Osborn of Sandia. “But the combination of tunable vacuum ultraviolet photons from the ALS and our new apparatus soon provided overwhelming evidence of this new chemical outcome that had been missed for decades.”
Like water, SO2 has a bent shape, with the sulfur atom in the middle. But how could the central atom be ejected from this stable molecule? Theoretical studies showed that the system first rearranges from the O-S-O structure to an S-O-O structure, after which the S departs, leaving behind O2.
“This photochemical reaction can’t happen in Earth’s lower atmosphere today because our ozone layer thankfully shields us from 193-nm light,” said Osborn. “However, for the first 2 billion years of Earth’s existence, there was very little atmospheric oxygen, and therefore no ozone. So this harsh 193-nm light penetrated to Earth’s surface, where it could break apart SO2 and directly form some of the O2 that we breathe today.”
The best evidence that the Great Oxygenation Event occurred comes from sulfur atoms in rock layers that are linked to how ancient sunlight dissociated SO2. However, there are still discrepancies in this theory. Including this new source of S and O2 into models of Earth’s early atmosphere could help reconcile these discrepancies and lead to a better understanding of how Earth developed its oxygen-rich atmosphere.
D. Rösch, Y. Xu, H. Guo, X. Hu, and D.L. Osborn, “SO2 Photodissociation at 193 nm Directly Forms S(3P) + O2(3Σg–): Implications for the Archean Atmosphere on Earth,” J. Phys. Chem. Lett. 14, 3084 (2023).