A new technique developed at the ALS offers sub-nanometer depth resolution of every chemical element to be found at heterogeneous interfaces, such as those in batteries and fuel cells. The technique, Standing-Wave Ambient-Pressure Photoelectron Spectroscopy (SWAPPS), combines standing-wave photoelectron spectroscopy (SWPS) with high-ambient-pressure photoelectron spectroscopy (APPS). The result is a technique that enables researchers to study a host of surface chemical processes under realistic pressure conditions. The technique is very promising for measuring such interfaces, which are critical components in energy research, heterogeneous catalysis, electrochemistry, and atmospheric and environmental science.
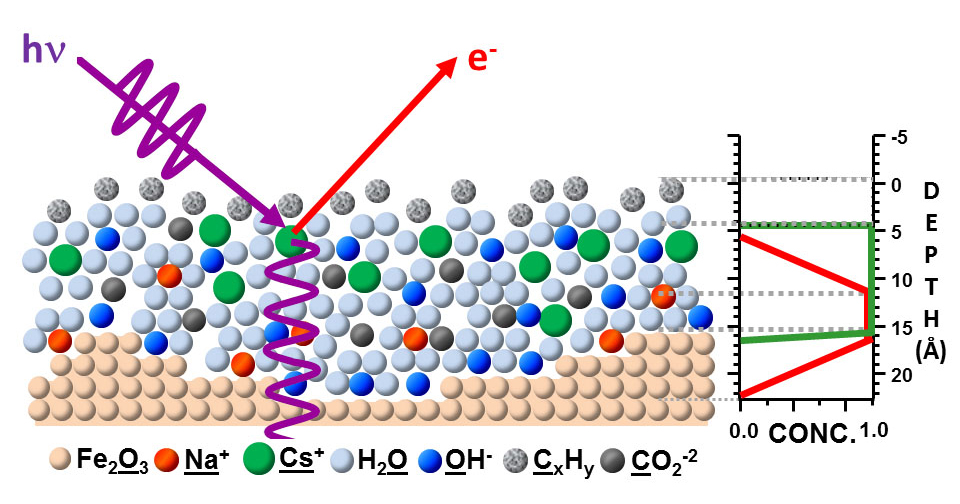
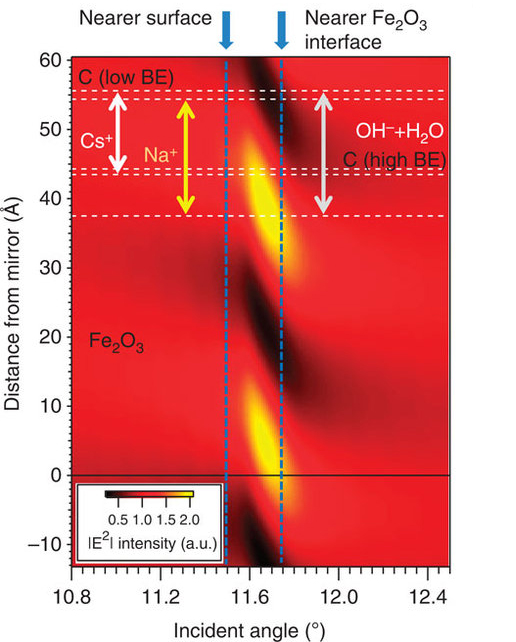
A key to the success of this study was the use of x-ray standing waves to excite the photoelectrons. A standing wave is an oscillatory pattern created when two waves of identical wavelength interfere with one another: one is the incident x-ray and the other is the x-ray reflected by a mirror. By suitable rotation of the sample, the x-ray standing wave can be scanned through the interface, with the photoelectrons excited by it revealing much about the depth distributions of each chemical species in a sample. Tailoring the x-ray wave field into a standing wave can thus be used to achieve much greater depth sensitivity in photoelectron spectroscopy.Solid/gas, liquid/gas, and solid/liquid interfaces play a major role in many important areas of science and technology, for example, in energy generation, electrochemistry, corrosion, environmental science, and information technology, but pose experimental challenges for the investigation of their chemical and structural properties on the molecular scale. The electrical double layer in batteries and fuel cells, for example, has been studied for over 100 years and yet is not fully understood. Characterizing such interfaces requires enhancing the signal from the narrow interfacial regions over those originating from the layers on either side of them.
SWAPPS therefore provides all the advantages of the widely used technique of x-ray photoelectron spectroscopy, including element and chemical-state sensitivity, and quantitative analysis of relative concentrations of all species present. However, SWAPPS does not require the usual ultrahigh-vacuum environment around the sample, which means researchers can measure the interfaces between volatile liquids and solids. SWAPPS can deliver vital information about the structure and chemistry of liquid/vapor and liquid/solid interfaces, in particular the electrical double layer.
ALS researchers have used SWAPPS to study a model system in which a nanometer layer of an aqueous electrolyte of sodium hydroxide and cesium hydroxide was grown on an iron oxide (hematite) solid. The spatial distributions of the electrolyte ions and the carbon contaminants across the solid/liquid and liquid/gas interfaces were directly probed and absolute concentrations of the chemical species were determined. The observation of binding-energy shifts with depth provided additional information on the bonding and/or depth-dependent potentials in the system.
They were able to determine that the sodium ions were located close to the iron oxide/solution interface, while cesium ions were on average not in direct contact with the solid/liquid interface. They also discovered two different kinds of carbon species, one hydrophobic, which is located exclusively in a thin film at the liquid/vapor interface, and a hydrophilic carbonate or carboxyl that is evenly distributed throughout the liquid film.
The SWAPPS combination of an oscillatory standing-wave field and the exponential decay of the photoelectron signal at each interface gives researchers unprecedented depth resolution. Future time-resolved SWAPPS studies using free-electron laser or high-harmonic generation light sources would also permit, via pump-probe methods, looking at the time scales of processes at interfaces on the femtosecond time scale. Many future applications of this technique thus seem possible.
Contact: Charles Fadley
Research conducted by: S. Nemšák, C. Conlon, A. Keqi, A. Rattanachata, and C.S. Fadley (UC Davis, Berkeley Lab); A. Shavorskiy, O. Karslioglu, I. Zegkinoglou, F. Salmassi, E. Gullikson, and H. Bluhm (Berkeley Lab); P. Greene, E. Burks, K. Liu (UC Davis), S. Yang (IBM Almaden Research Center).
Research funding: U.S. Department of Energy (DOE), Basic Energy Sciences (BES). Operation of the ALS is supported by the DOE BES.
Publication about this research: S. Nemšák, C. Conlon, A. Keqi, A. Rattanachata, C.S. Fadley, A. Shavorskiy, O. Karslioglu, I. Zegkinoglou, F. Salmassi, E. Gullikson, H. Bluhm, P. Greene, E. Burks, K. Liu, and S. Yang, “Concentration and chemical-state profiles at heterogeneous interfaces with sub-nm accuracy from standing-wave ambient-pressure photoemission,” Nature Communications 5, 5441 (2014). doi:10.1038/ncomms6441
ALS SCIENCE HIGHLIGHTS #306