The structure of liquid water has been intensely studied, but until recently, it has not been clear what happens to it when a surface is introduced. ALS researchers have now made a first-ever observation of the molecular structure of liquid water at a gold surface under different charging conditions. This marks the first time that the scientific community has shown such high sensitivity in an in-situ environment under working electrode conditions.
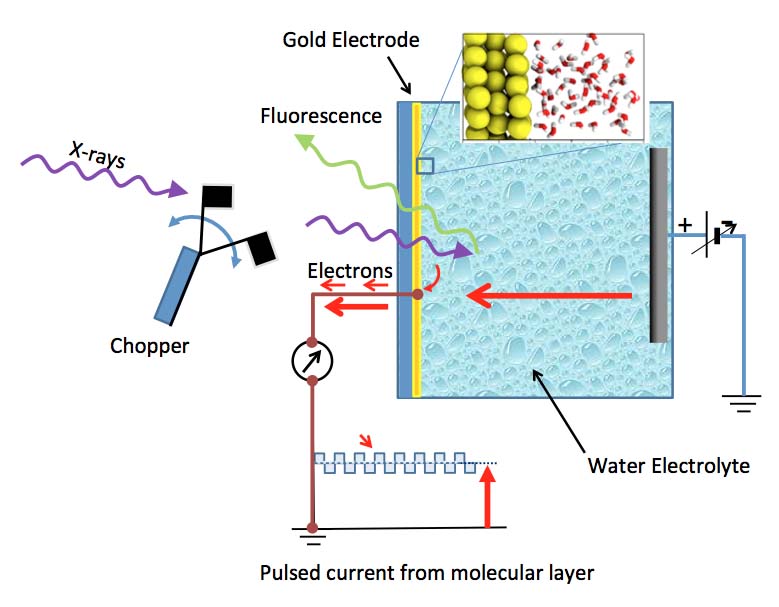
The structure of water within a nanometer of an electrode surface is known as the electrical double layer. The strong electrical field that builds up across the double layer is what drives electrochemical reactions. ALS researchers explored the structure of the solvent structure in a aqueous electrical double later at a bare gold electrode. With no applied potential and at positive potentials, the layer is highly structured (resembling ice), with few dangling hydrogen bonds. However, at negative potentials, the layer is more like bulk water, but with half of the water molecules lying roughly flat on the surface.
ALS researchers were able to develop a method not only to look at the molecules next to the electrode surface, but to determine their arrangement changes depending on the voltage. With gold as a chemically inert electrode, and slightly saline water as an electrolyte, the researchers used a new twist on x-ray absorption spectroscopy (XAS) to probe the interface and show how the interfacial molecules are arranged.
Upon absorbing an x-ray photon, the excited water molecule can emit either electrons or photons. The amount of photon emission, or fluorescence, is one indicator of how many x-ray photons have been absorbed. However, fluorescing x-rays can be detected from molecules ranging from those at the gold surface to those deep (micrometers) inside the liquid far from the influence of the gold surface, and these dominate the measured spectrum. The challenge was to collect a signal that would be dominated by the interfacial region. Researchers accomplished this by measuring electron emissions because electrons emitted from x-ray excited water molecules travel only nanometer distances through matter.
There’s an additional problem that arises when studying liquids in contact with working electrodes because they carry a steady current as in batteries and other electrochemical systems. While the emitted electrons from nearby molecules are detectable, this contribution to the current is dwarfed by the normal “Faradaic” current of the battery at finite voltages. When measuring current off the electrode, it is critical to determine which part is due to the x-rays and which is due to the regular battery current.
To overcome this problem, the researchers pulsed the incoming x-rays from the synchrotron at a known frequency. The current contribution resulting from electron emission by interfacial molecules is thus pulsed as well, and instruments can separate this nanoampere modulated current from the main Faradaic current.
These experiments result in absorption vs. x-ray energy curves (spectra) that reflect how water molecules within nanometers of the gold surface absorb the x-rays. To translate that information into molecular structure, a theoretical analysis technique was developed by scientists at Berkeley Lab’s Molecular Foundry.
It turns out that for a neutral gold surface, a significant number of water molecules next to the gold surface orient with hydrogen (H) atoms pointing toward the gold. Water molecules are bound together by hydrogen bonds, which orient the slightly positively charged H atoms in each molecule towards the slightly negatively charged oxygen (O) atoms of neighboring molecules. This network of hydrogen bonds is what holds water molecules together to make a liquid under conditions of temperature and pressure that we consider comfortable as humans. It is perhaps surprising that the inert gold surface can induce significant numbers of water molecules not to hydrogen-bond to each other but to bond to the gold instead. This number is enhanced when the gold is negatively charged and therefore attracting the more positive H atoms. Furthermore, positively charged gold ions cause water molecules to orient their H atoms away from the gold, which strengthens the hydrogen bond network of the interfacial liquid.

Contact: Miquel Salmeron
Research conducted by: J.Velasco-Velez, T.A. Pascal, L.F. Wan, and D. Prendergast (Berkeley Lab); C.H. Wu and M. Salmeron (Berkeley Lab, UC Berkeley); and J.-H. Guo (Berkeley Lab).
Research funding: U.S. Department of Energy (DOE), Basic Energy Sciences (BES). Operation of the ALS is supported by the DOE BES.
Publication about this research: J.Velasco-Velez, C. Wu, T.A. Pascal, L.F. Wan, J.-H. Guo, D. Prendergast, and M. Salmeron, “The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy,” Science 346, 831 (2014). doi:10.1126/science.1259437
ALS SCIENCE HIGHLIGHT #308