SCIENTIFIC ACHIEVEMENT
Researchers from Genentech used a suite of methods, including small-angle x-ray scattering at the Advanced Light Source (ALS), to learn how an assembly of three proteins works together to transmit signals for cell division.
SIGNIFICANCE AND IMPACT
The work reveals new targets for the development of drugs that fight certain types of cancer, including lung, colorectal, and pancreatic cancer.
Advances in fighting cancer
Great progress has recently been made in understanding the cascade of molecular signals that trigger cell replication, and compounds that block these signals when they run amok are of great interest as potential cancer treatments. For example, a promising anticancer drug, currently in clinical trials for the treatment of non-small cell lung cancer, inhibits tumor growth by locking a signaling protein in an inactive state.
While such drugs have shown efficacy in the clinic, resistance mechanisms that reactivate the signaling pathway have emerged. Thus, it is vitally important to gain a molecular-level understanding of the various ways in which signaling proteins interact with each other. To that end, a research group from Genentech has used a suite of methods, including small-angle x-ray scattering (SAXS) at the ALS, to gain insight into a signaling pathway implicated in lung, colorectal, and pancreatic cancer.
The RAS-to-RAF relay
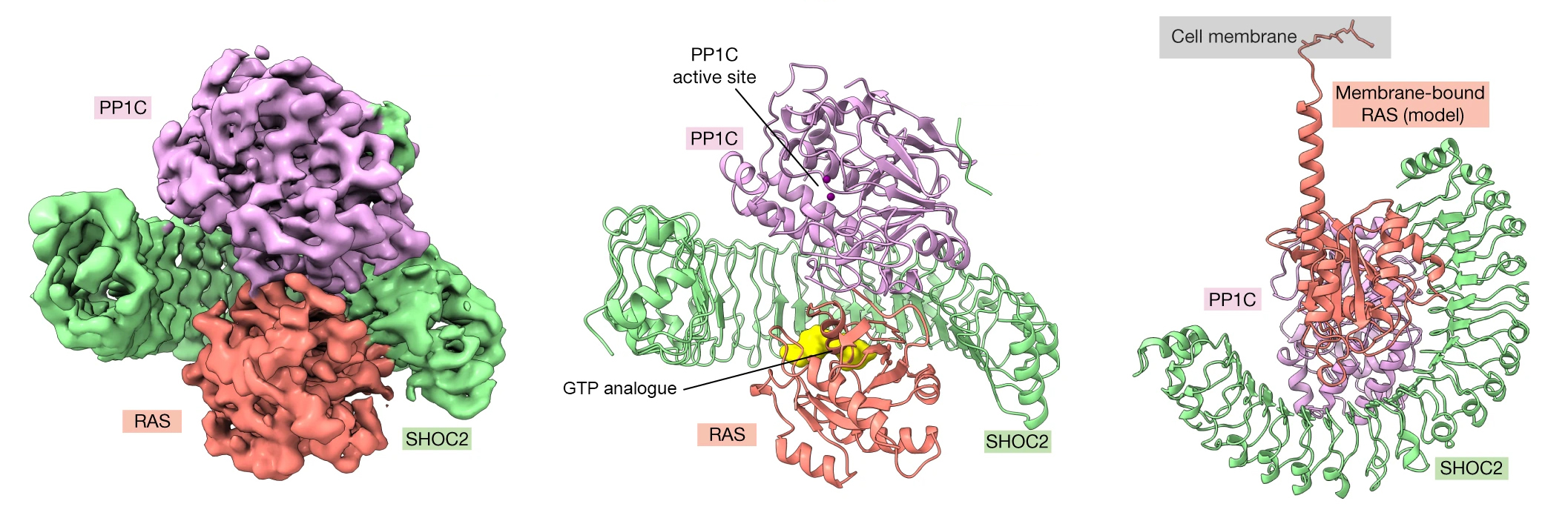
The initiation of cell division requires a long chain of molecular interactions. Here, the focus was on a particular sequence in which the signal is relayed between two proteins known as RAS and RAF. The signaling begins with the attachment of a growth-factor molecule to a cell’s outer surface. This causes RAS proteins on the inside of the cell membrane to bind GTP, a small molecule that activates many biological processes. A GTP-loaded RAS can then form a complex with a crescent-shaped scaffold protein (SHOC2) and an enzyme (PP1C) that dephosphorylates (removes a phosphate group from) other proteins. Once assembled, the SHOC2-PP1C-RAS complex is able to dephosphorylate its target: the RAF protein. The dephosphorylated RAF is now able to form a dimer, a prerequisite to proceeding on to the next step in the signal cascade.
Despite decades of study, the molecular details underlying the dimerization of RAF remain unclear. The Genentech research group had previously used the ALS to study competing mechanisms involved in RAF dimerization. In this work, they sought to understand the molecular basis for the formation of the SHOC2-PP1C-RAS complex, through biochemical and structural studies.
Multimodal structure studies
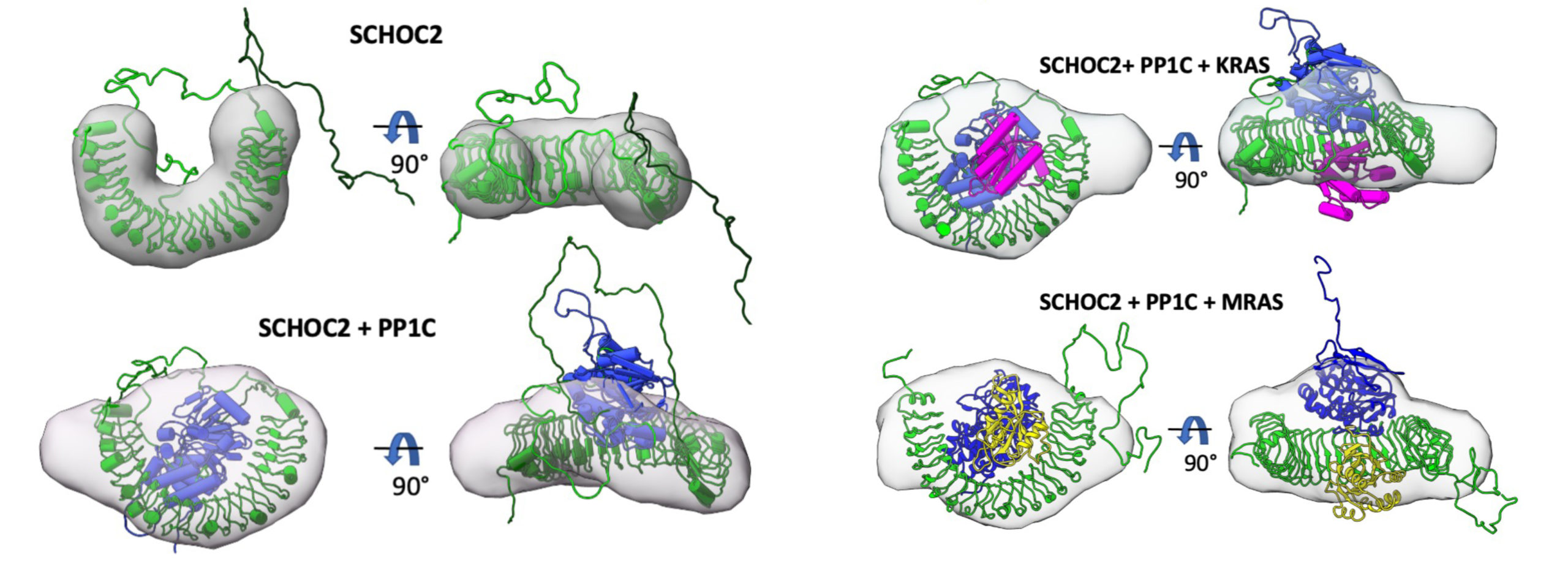
The researchers used cryogenic electron microscopy (cryoEM), protein crystallography at Stanford Synchrotron Radiation Laboratory (SSRL), and SAXS studies at ALS Beamline 12.3.1 (SIBYLS) to structurally characterize the SHOC2-PP1C-RAS complex. SAXS captures snapshots of large, flexible protein complexes in their native form (suspended in solution), enabling the researchers to visualize dynamic aspects of complex formation. At the beamline, samples were also analyzed using size exclusion chromatography (SEC) and multiangle light scattering (MALS). This high-throughput SEC-SAXS-MALS capability was important for validating various SHOC2-PP1C-RAS assemblies prior to cryoEM studies.
Using the SAXS data, the researchers were able to model the conformational flexibility of the SHOC2 N-terminal region and determine its contribution to complex formation. Together with the other structural data, biochemical studies, and molecular dynamics simulations, the work answered many outstanding questions about RAS-RAF signaling, including how disease-relevant mutations affect assembly of the complex and how the SHOC2 scaffold and RAS collectively drive PP1C to target RAF for dephosphorylation. In general, the work establishes fresh avenues for the rational discovery of new classes of inhibitors targeting the RAS-RAF pathway.
Contact: Michal Hammel
Researchers: N.P.D. Liau, M.C. Johnson, S. Izadi, L. Gerosa, J.M. Bruning, T.J. Wendorff, W. Phung, S.G. Hymowitz, and J. Sudhamsu (Genentech); and M. Hammel (Berkeley Lab).
Funding: U.S. Department of Energy (DOE), Office of Science, Biological and Environmental Research program; National Institutes of Health; and Genentech. Operation of the ALS and SSRL is supported by DOE, Office of Science, Basic Energy Sciences program.
Publication: N.P.D. Liau, M.C. Johnson, S. Izadi, L. Gerosa, M. Hammel, J.M. Bruning, T.J. Wendorff, W. Phung, S.G. Hymowitz, and J. Sudhamsu, “Structural basis for SHOC2 modulation of RAS signalling,” Nature 609, 400 (2022), doi:10.1038/s41586-022-04838-3.
ALS SCIENCE HIGHLIGHT #471