Understanding how materials absorb and release hydrogen is the focus of the Hydrogen Materials Advanced Research Consortium (HyMARC). At the ALS, the HyMARC Approved Program was recently renewed, underscoring the key role that soft x-ray techniques have played in addressing the challenges of hydrogen storage. Read more »
Spectroscopic investigation of a Co(0001) model catalyst during exposure to H2 and CO at near-ambient pressures
We have performed near-ambient-pressure X-ray photoelectron spectroscopy on Co(0001) model catalysts during exposure to gases relevant to Fischer–Tropsch synthesis, i.e., CO and H2, at 0.25 mbar total pressure. At this pressure, CO seems to be more efficient at keeping the Co(0001) surface metallic than H2, which is the opposite behavior as reported in the literature for other pressure ranges. Read more »
Synergistic Effect Could Boost Production of Green Hydrogen
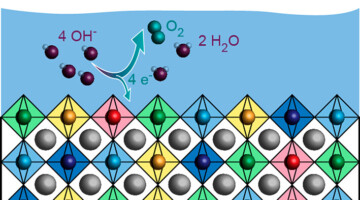
Researchers developed a composite material of earth-abundant elements that catalyzes the production of green hydrogen much more effectively than similar homogeneous compounds. The composite could potentially be used for efficient hydrogen generation without the need for rare and precious metals like platinum. Read more »
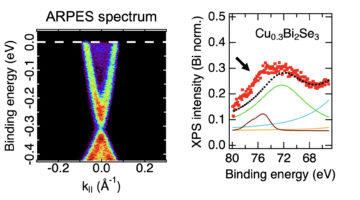
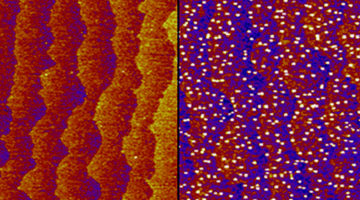
Copper Migrates to Surface of Topological Insulator in Air
An ambient-pressure study of a topological insulator doped with copper revealed that the copper atoms, inserted between the material’s layers, migrate to the surface when exposed to air. The work represents a novel way of modifying the material’s surface composition, which can confer it with new properties such as superconductivity. Read more »
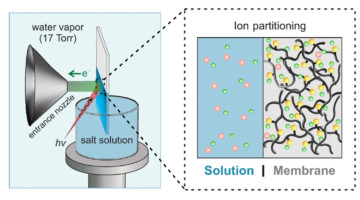
First Direct Measurement of Elusive Donnan Potential
Researchers performed the first direct measurement of the Donnan electrical potential, which arises from an imbalance of charges at membrane-solution interfaces. Considered unmeasurable for over a century, the Donnan potential is relevant to a wide range of fields, from cell biology to energy storage and water desalination. Read more »![]()
![]()
The Donnan Potential, Revealed at Last
Researchers at the ALS recently led the first direct measurement of the Donnan potential, an electric potential that arises from an imbalance of charges at the interface of a charged membrane and a liquid. The work could yield new insights in areas such as ion transport through cellular membranes, ion exchange membranes in energy storage strategies, and water purification technologies. Read more »
Watching Nanoparticle Chemistry and Structure Evolve
Using a multimodal approach, researchers learned how chemical properties correlate with structural changes during nanoparticle growth. The work will enable a greater understanding of the mechanisms affecting the durability of nanoparticles used to catalyze a broad range of chemical reactions, including clean-energy reactions. Read more »![]()
![]()
Highly Selective Methane to Methanol Conversion on Inverse SnO2/Cu2O/Cu(111) Catalysts: Unique Properties of SnO2 Nanostructures and the Inhibition of the Direct Oxidative Combustion of Methane
Inverse catalysts generally consist of oxide nanoparticles supported on metal substrates, which can exhibit exceptional catalytic properties. The small SnO2 nanoparticles uniformly dispersed on a Cu2O/Cu(111) substrate enabled a unique SnO2–Cu2O interface that can completely convert methane to methanol directly under the environments of oxygen and water. Read more »
A Photoelectrode Protection Scheme for Solar-Fuel Production
Microscopy, spectroscopy, and computational studies of a promising artificial-photosynthesis material led researchers to develop a model photoelectrochemical (PEC) cell with remarkable stability and longevity as it selectively converts sunlight and carbon dioxide into two promising sources of renewable fuels—ethylene and hydrogen. Read more »
New Device Advances Commercial Viability of Solar Fuels
A Berkeley Lab research team developed a new artificial photosynthesis device component that exhibits remarkable stability and longevity as it selectively converts sunlight and carbon dioxide into two promising sources of renewable fuels—ethylene and hydrogen. Read more »