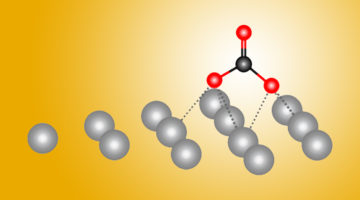
Combining ALS experiments with quantum-mechanical calculations, scientists found dramatic differences in how carbon dioxide (CO2) reactions begin on silver as opposed to copper. Both metals help transform CO2—a greenhouse gas—into more useful forms, and this new atomic-level data could help make the process more efficient. Read more »![]()
![]()
Fuel from the Sun: Insight into Electrode Performance
The mechanisms limiting the performance of hematite electrodes—potentially key components in producing fuel from the sun—have been clarified in interface-specific studies under realistic operating conditions, bringing us a step closer to storing solar energy in chemical fuels. Read more »![]()
![]()
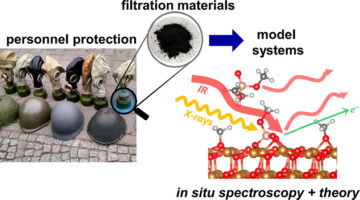
Studying Gas Mask Filters So People Can Breathe Easier
Scientists have put the x-ray spotlight on composite materials in respirators used by the military, police, and first responders. The results provide reassuring news about the effectiveness of current filters and provide fundamental information that could lead to more advanced gas masks as well as protective gear for civilian applications. Read more »
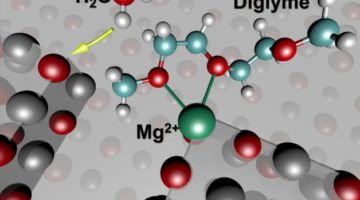
Scientists Solve a Magnesium Mystery in Rechargeable Battery Performance
Rechargeable batteries based on magnesium, rather than lithium, have the potential to pack more energy into smaller batteries. However, researchers have discovered a surprising set of chemical reactions involving magnesium that degrade battery performance even before the battery can be charged up. Read more »
CO Adsorption on Pd(100) Studied by Multimodal Ambient Pressure X-Ray Photoelectron and Infrared Reflection Absorption Spectroscopies
The first combined infrared spectroscopy and ambient-pressure XPS study was demonstrated at Beamline 11.0.2. The in situ vibrational and core-level spectroscopies in the Torr pressure range offer complementary information on the properties of surfaces and adsorbates while closing the pressure gap between laboratory measurements and applications. The multimodal spectroscopy also allowed the identification of the C 1s binding energy and quantification of an uncommon atop CO species on a Pd(100) surface. Read more »
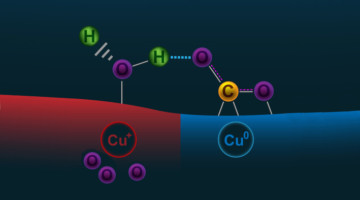
Subsurface Oxygen Boosts Activity of Copper Catalysts
Scientists are seeking ways to reduce levels of CO2 in the atmosphere by improving the processes that convert CO2 gas into ethanol (a liquid fuel). But copper, the best catalyst for this, is not very efficient. Now, ambient-pressure x-ray experiments have revealed how subsurface oxygen boosts copper’s catalytic activity. Read more »![]()
![]()
A Closer Look at Dynamic Restructuring in Catalysts
Researchers have structurally and chemically “visualized” the surface of a silver–gold alloy as it reorganizes itself during catalytic activation. The insights gained from this methodology can lead to improved catalysts for energy-intensive industrial applications, thereby increasing efficiency and reducing waste. Read more »![]()
![]()
Researchers Find a Surprise Just Beneath the Surface in Carbon Dioxide Experiment
X-ray experiments, coupled with theoretical work, revealed how oxygen atoms embedded near the surface of a copper sample had a more dramatic effect on the early stages of a reaction with CO2 than accounted for in earlier theories. This work could prove useful in designing new catalysts for converting CO2 into liquid fuels and other products. Read more »
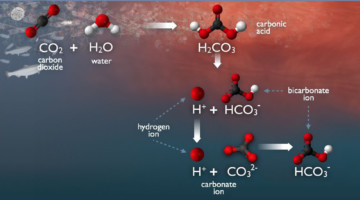
APXPS Finds Carbonate Reversal at Liquid Interfaces
Aqueous carbonate systems are central to many processes essential to life, from the blood buffer system to the global carbon cycle. Using APXPS, researchers probed the concentration of carbonates near an interface, finding a surprising reversal in the expected abundances as a function of depth. Read more »![]()
![]()
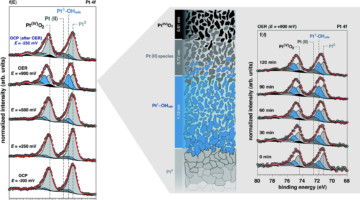
A Closer Look at a Working Platinum/Electrolyte Interface
Ambient-pressure studies of the interface between a platinum electrode and an alkaline electrolyte revealed the molecular-level chemistry, structure, and dynamics of the platinum surface as a function of applied potential, highlighting differences between thermodynamic predictions and the actual surface composition. Read more »