Scientists discovered an ancient form of rubisco, the most abundant enzyme on Earth and critical to life as we know it. Found in previously unknown environmental microbes, the newly identified rubisco provides insight into the evolution of the photosynthetic organisms that underlie the planet’s food chains. Read more »
Providing New Technologies for Vaccine Development
Antigens can sometimes be attached to a protein scaffold to mimic the shape of a virus and elicit a stronger immune response. Scientists developed a method to design such proteins, and ALS data helped to visualize the atomic structure and determine the dynamics of the designed scaffolds. Read more »
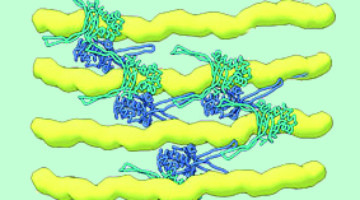
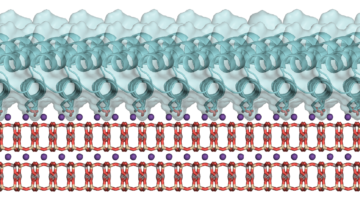
How Proteins Remodel DNA in Bacteria under Stress
Multiscale, multimodal visualization techniques at the ALS enabled researchers to clarify how proteins remodel bacterial DNA in response to stressful environments. The discovery could lead to new strategies for controlling microbial behavior and, eventually, new ways to fight bacterial infections. Read more »![]()
![]()
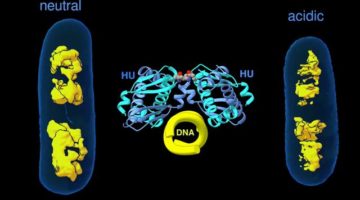
Study Gains New Insight Into Bacterial DNA Packing
When bacteria are put in different environments, their genes start to adapt remarkably quickly because the proteins making up their chromosomes can pack and unpack rapidly. Researchers have now imaged this process at the molecular level, a discovery that could eventually enable scientists to develop strategies to control microbial behavior. Read more »
This Enigmatic Protein Sculpts DNA to Repair Harmful Damage
Scientists have discovered that a DNA-repairing protein performs its functions by first marking and then further breaking damaged DNA. The surprising findings have provided much-needed insight into how DNA repair works in healthy human cells, as well as how different mutations can translate into different diseases and cancer. Read more »
Assembly Lines for Designer Bioactive Compounds
Researchers successfully bioengineered changes to a molecular “assembly line” for bioactive compounds, based in part on insights gained from small-angle x-ray scattering at the ALS. The ability to re-engineer these assembly lines could improve their performance and facilitate the synthesis of new medically useful compounds. Read more »![]()
![]()
X-Ray Technology Sheds New Light on Antibiotic Synthesis
Atomic-scale structural analyses performed at the ALS are helping scientists understand the inner workings of the enzyme “assembly lines” that microbes use to produce an important class of compounds, many of which have uses as antibiotics, antifungals, and immunosuppressants. Read more »
Structural Basis for Finding OG Lesions and Avoiding Undamaged G by the DNA Glycosylase MutY
Finding OG and avoiding G: DNA repair enzyme MutY distinguishes between undamaged guanine (green) and oxidized guanine when targeting OG:A mispairs. A structural motif within the C-terminal domain (violet) responds to OG to G substitution and appears mechanistically coupled to the adenine removal site (gray) in the N-terminal domain (cyan). Read more »
Custom-Designed Models Reveal How Proteins Assemble on Minerals
Seashells, bone, and other hard tissues form through a little-understood process combining proteins and minerals. Researchers gained insight using a model system of proteins they designed and synthesized from scratch, characterizing how these building blocks assemble on mica. Read more »
Exploring the “minimal” structure of a functional ADAMTS13 by mutagenesis and small-angle X-ray scattering
Researchers used the SIBYLS beamline to gain insight into ADAMTS13, the only known protein to regulate the adhesive function of von Willebrand factor (VWF), a blood-clotting protein. When VWF is deficient or abnormal, it causes a common inherited bleeding disorder, von Willebrand disease. VWF is also implicated in arterial and deep-vein thrombosis, stroke, atherosclerosis, sickle cell crisis, and sepsis. Read more »