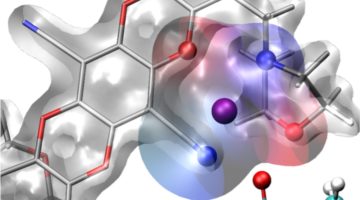
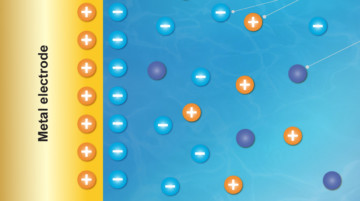
Researchers designed a polymer membrane with molecular cages built into its pores that hold positively charged ions from a lithium salt. These “solvation cages” increased lithium-ion flow by an order of magnitude and could allow high-voltage battery cells to operate at higher power and more efficiently, important for both electric vehicles and aircraft. Read more »
Probing Composite Materials to Make Better Batteries
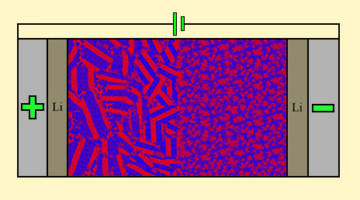
Researchers found that when an ion-conducting polymer composite is placed in an electric field, it forms ion-rich hotspots that continue to grow for hours after the field is removed. The study opens a new path to understanding the dynamic structure of composite materials for smaller, lighter batteries. Read more »![]()
![]()
Go With the Flow: Scientists Design Better Batteries for a Renewable Energy Grid
Researchers developed a versatile yet affordable battery membrane—from a class of polymers known as AquaPIMs. This class of polymers makes long-lasting and low-cost grid batteries possible based solely on readily available materials such as zinc, iron, and water. Read more »
Renewed Prospects for Rechargeable Mg Batteries
Contrary to previous reports, it’s possible to create a rechargeable battery using magnesium ions if the electrode material is first conditioned at high temperature. With twice the charge of lithium ions, magnesium ions hold great promise as the basis for high-energy-density batteries suitable for use in electric vehicles. Read more »![]()
![]()
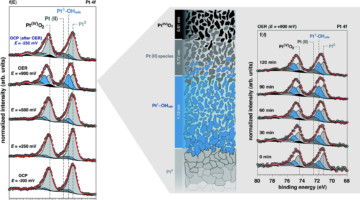
A Closer Look at a Working Platinum/Electrolyte Interface
Ambient-pressure studies of the interface between a platinum electrode and an alkaline electrolyte revealed the molecular-level chemistry, structure, and dynamics of the platinum surface as a function of applied potential, highlighting differences between thermodynamic predictions and the actual surface composition. Read more »
Tender X-Rays Map the Double-Layer Potential
In a first-of-its-kind experiment, ALS researchers demonstrated a new, direct way to study the inner workings of a phenomenon in chemistry known as an “electrochemical double layer” that forms where liquids meet solids—where battery fluid meets an electrode, for example. Read more »![]()
![]()