SCIENTIFIC ACHIEVEMENT
Researchers used the Advanced Light Source (ALS) to help show that, contrary to previous reports, it’s possible to create a rechargeable battery using magnesium ions if the electrode material is first conditioned at high temperature.
SIGNIFICANCE AND IMPACT
With twice the charge of lithium ions, magnesium ions hold great promise as the basis for high-energy-density batteries suitable for use in electric vehicles.
The driving motivation
Improved rechargeable batteries could go a long way toward lowering barriers to more widespread adoption of electric vehicles by consumers. In turn, greater use of electric vehicles could help control greenhouse-gas emissions and reduce the need for foreign oil.
A major consideration in the development of batteries for electric vehicles is the need to store a lot of energy in a small volume without adding significant weight. In other words, battery materials need to have a high energy density. So far, lithium-ion batteries have been the best practical option; in theory, however, other materials have the potential to do better.
Multivalent magnesium
In a rechargeable lithium-ion battery, singly charged lithium ions (Li1+) move back and forth between electrodes as the battery charges and discharges. One way to increase energy density is by using multivalent ions (ions having a charge higher than 1+). A magnesium ion (Mg2+) is not much bigger than a lithium ion, but it has two positive charges versus lithium’s one. Magnesium is also more abundant than lithium, which could result in lower costs.
However, the higher charge makes it more difficult to intercalate (embed) magnesium into an electrode, for electrostatic reasons. Moreover, recent studies involving a typical electrode material, vanadium oxide (V2O5), have cast doubt on the level of magnesium-ion intercalation that can be achieved, suggesting that past observations of electrochemical activity in such systems were actually due to proton intercalation instead.
Turning up the heat
In this work, researchers revisited the question of whether magnesium intercalation into vanadium oxide is inherently (thermodynamically) possible. To do this, they used a highly stable electrolyte that allowed experiments to be run at high temperature (110 °C). Using a multimodal approach that included scanning transmission x-ray microscopy (STXM) at ALS Beamline 5.3.2.2, the researchers probed the elemental, chemical, and structural changes in the vanadium oxide electrode at different stages of the charge-discharge cycle.
The results showed that vanadium oxide can indeed intercalate magnesium at high temperature, with minimal incorporation of other species, including protons. Strikingly, the experiments also revealed that the charge-discharge cycle could be repeated many times at room temperature (25 °C), something that doesn’t happen without the initial high-temperature cycle.
Conditioning the electrode
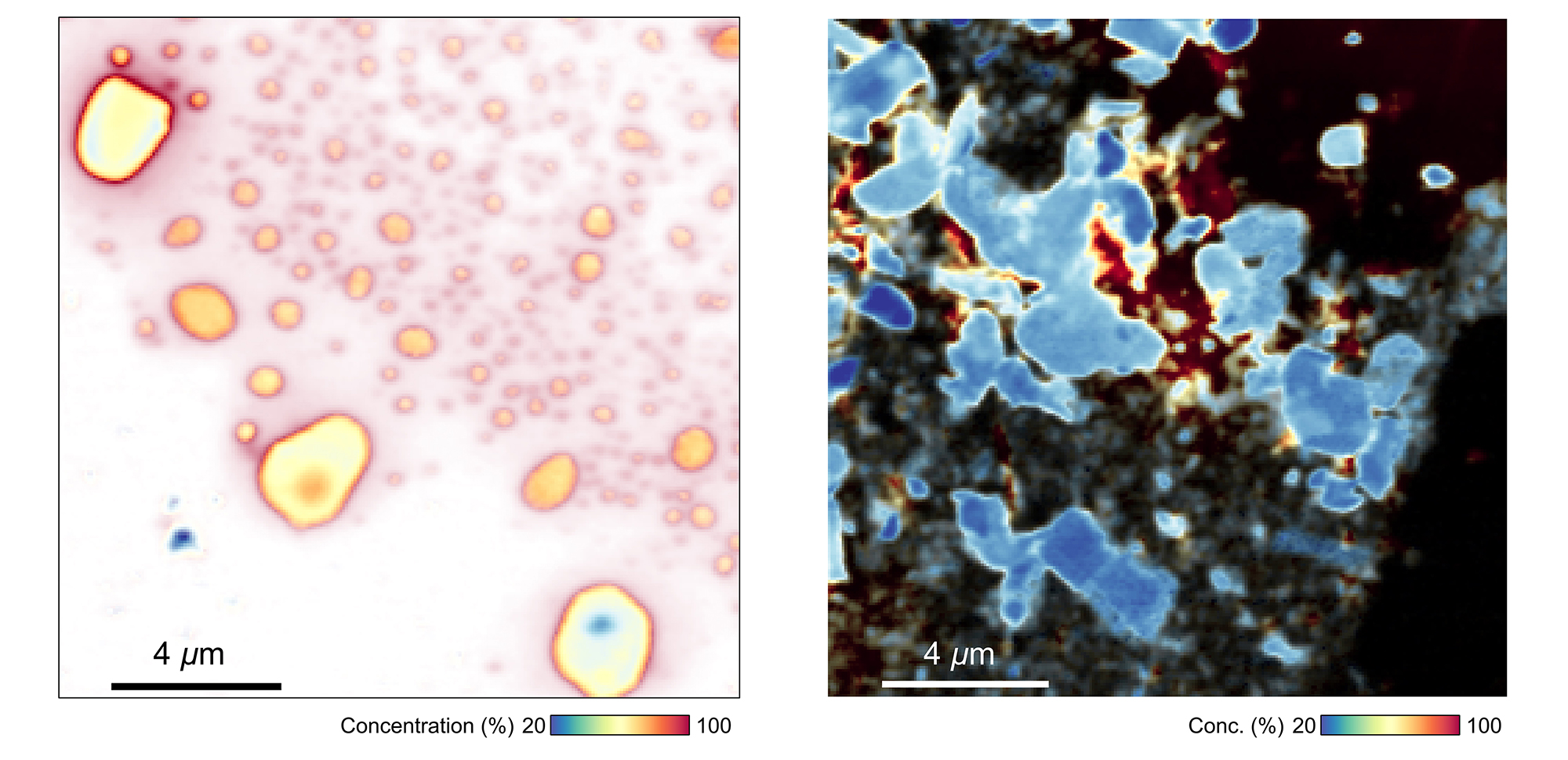
To explain this phenomenon, the researchers used STXM data to locate where magnesium was concentrated within the electrode particles. After discharge (when the electrode hosts the magnesium ions), the maps showed that magnesium concentrations were highest at surfaces and around cracks, with smaller particles being more active. Based on this observation, the researchers concluded that driving the magnesium ions into the vanadium oxide at high temperature helps break up the material, increasing the accessible surface area and making it easier for the reaction to proceed at room temperature.
In future experiments, the researchers hope to learn what initial particle size would remove the need for high-temperature conditioning and, more generally, to gain further insight into solutions for other bottlenecks in the reaction. In the meantime, these findings represent an important step toward the future design of multivalent batteries that transcend the limitations of lithium-ion batteries.
Contact: Jordi Cabana
Researchers: H.D. Yoo (Univ. of Illinois at Chicago, Argonne National Laboratory, and Pusan National University, Republic of Korea); J.R. Jokisaari, B.J. Kwon, L. Hu, G.M. Nolis, R.F. Klie, and J. Cabana (Univ. of Illinois at Chicago and Argonne National Laboratory); Y.-S. Yu (ALS); S. Kim, S.-D. Han, M. Lopez, S.H. Lapidus, B.J. Ingram, S. Ahmed, J.T. Vaughey, and T.T. Fister (Argonne National Laboratory); and I.L. Bolotin (Univ. of Illinois at Chicago).
Funding: U.S. Department of Energy (DOE), Joint Center for Energy Storage (JCESR); University of Illiniois at Chicago; National Science Foundation; DOE Office of Science, Basic Energy Sciences Program (BES); and Korean Ministry of Science and ICT. Operation of the ALS is supported by the DOE BES.
Publication: H.D. Yoo, J.R. Jokisaari, Y.-S. Yu, B.J. Kwon, L. Hu, S. Kim, S.-D. Han, M. Lopez, S.H. Lapidus, G.M. Nolis, B.J. Ingram, I.L. Bolotin, S. Ahmed, R.F. Klie, J.T. Vaughey, T.T. Fister, and J. Cabana, “Intercalation of Magnesium into a Layered Vanadium Oxide with High Capacity,” ACS Energy Lett. 4, 1528 (2019), doi:10.1021/acsenergylett.9b00788.
ALS SCIENCE HIGHLIGHT #402