Ignition chemistry involves a complex web of reactions. By studying the reaction products—looking at this web from its perimeter inward—researchers have gradually deduced the nature of the “reactive intermediate” molecules that must lie at the center. Decades of research worldwide have shown that hydroperoxyalkyl radicals—a class of reactive molecules denoted as “QOOH”—must be a central connection in this network. At the ALS, researchers from Sandia National Laboratories have directly observed QOOH molecules for the first time. This breakthrough has generated data on QOOH reaction rates that will improve the fidelity of models used by engine manufacturers to create cleaner and more efficient cars and trucks.
Nearly 10 years ago, Sandia researchers designed a new instrument, the Multiplexed Photoionization Mass Spectrometer (MPIMS), to directly probe all kinds of intermediates, including the species that are at the center of important webs of reactions. In 2012, the Sandia team, together with colleagues from the University of Manchester and Bristol University in England, used the MPIMS to directly measure reaction rates and products of the “Criegee intermediate,” a crucial reactive molecule in the web of reactions that occur in atmospheric chemistry. QOOH was next in line.
But even with processes and tools in place, creative thinking was called for. They needed a specialized strategy to create enough QOOH radicals to detect, and they needed to determine the spectral fingerprint of a QOOH molecule, so that they would recognize it if they created it. John Savee, a postdoctoral chemist at Sandia, hypothesized that the best fuel for producing a detectable QOOH is cycloheptadiene, a molecule with seven carbon atoms arranged in a ring. Initial experiments seemed to prove the idea was right, and the team turned to its computational experts, who used quantum chemistry to predict what the experimentalists should observe.
For the most definitive measurements, the team moved the MPIMS to ALS Chemical Dynamics Beamline 9.0.2, where they performed photoionization mass spectrometry on cycloheptadiene combustion products at a variety of temperatures and concentrations of O2. The intense, tunable, vacuum ultraviolet light created by the synchrotron allowed the team to measure spectral fingerprints of molecules, deducing the particular arrangement of atoms that gives a molecule its identity. They confirmed that the spectrum of the radical they observed matched computational predictions, showing that it was in fact a QOOH molecule, rather than some other possible arrangement of the same atoms.
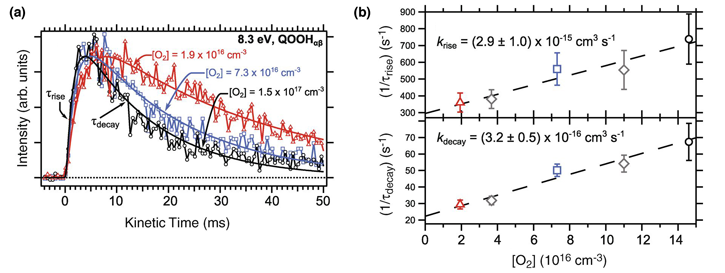
The particular QOOH radical the team detected has a relatively long lifetime, reacting much more slowly with oxygen than any previous estimates. The impact of this class of QOOH radicals, which the team predicts will all have long lifetimes, is not yet clear, and their data will be incorporated into the latest combustion models to test its impact. Interestingly, the same class of QOOH radicals has recently been proposed as a key intermediate that converts hydrocarbons in the atmosphere into small aerosol particles that impact health, visibility, and climate. Present models of atmospheric aerosol formation can’t match the rate and size growth of these particles, and the QOOH intermediate may help bring observations and models into agreement.
The researchers acknowledge there is still much to do to create a complete and accurate model of ignition or atmospheric oxidation. For example, measurements of other, more reactive QOOH species will be important for predicting ignition and oxidation behavior of a range of fuels. “We know from our experience with the Criegee intermediate that researchers around the world will make great use of this information,” said principal investigator David Osborn. “And because these oxidation processes are important in many areas, including atmospheric studies, the impacts are likely to reach far beyond combustion.”
Contact: David Osborn
Research conducted by: J.D. Savee, E. Papajak, B. Rotavera, H. Huang, A.J. Eskola, O. Welz, L. Sheps, C.A. Taatjes, J. Zádor, and D.L. Osborn (Sandia National Laboratories).
Research funding: U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES). Operation of the ALS is supported by DOE BES.
Publication about this research: J.D. Savee, E. Papajak, B. Rotavera, H. Huang, A.J. Eskola, O. Welz, L. Sheps, C.A. Taatjes, J. Zádor, and D.L. Osborn, “Direct observation and kinetics of a hydroperoxyalkyl radical (QOOH),” Science 347, 643 (2015).
ALS SCIENCE HIGHLIGHT #315