Rechargeable lithium-ion batteries work by shuttling lithium ions between electrodes as the battery charges and discharges. If more lithium ions could be inserted into the cathode during discharge, the battery’s capacity would be greater for a given battery size and weight—i.e., it could last longer between recharges and/or run more power-thirsty applications.
Thus, researchers are interested in materials such as vanadyl phosphate, which can theoretically accept twice the number of lithium ions as cathode materials currently in use, all while remaining compatible with existing battery architecture and having better thermal and chemical stability. However, preliminary tests have shown that, in practice, vanadyl phosphate doesn’t live up to expectations, accepting fewer than the optimal two lithium ions per active site.
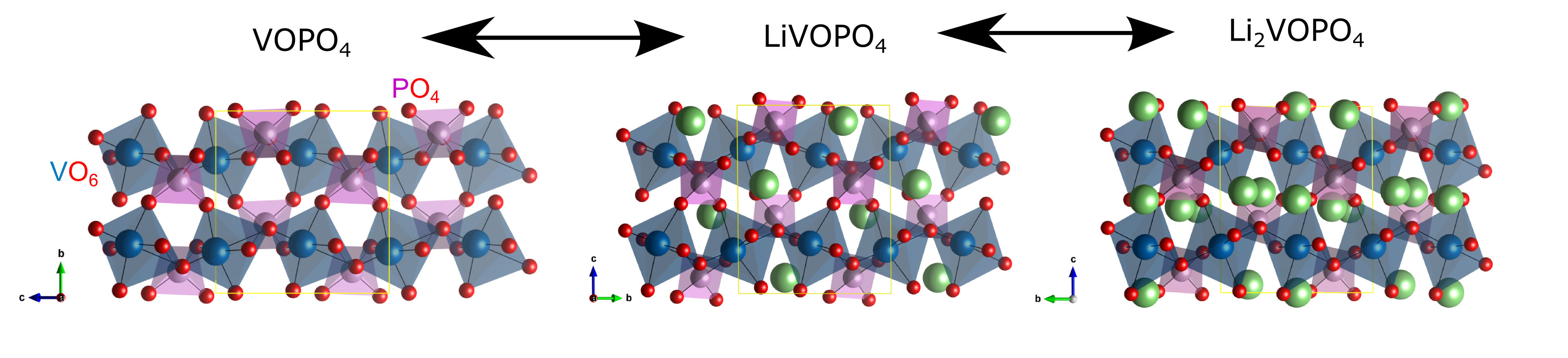
At ALS Beamline 6.3.1, researchers from the NorthEast Center for Chemical Energy Storage (NECCES) Energy Frontier Research Center (EFRC) studied ϵ-LiVOPO4, a form of vanadyl phosphate that already includes one lithium ion in each active site. Using a variety of hard and soft x-ray spectroscopies, the researchers looked at the vanadium oxidation state at the surface, subsurface, and bulk, at various stages of charge and discharge.
They found that ϵ-LiVOPO4 gets much closer to the two-lithium capacity throughout the whole cathode uniformly, and that capacity degrades significantly during the transition between the insertion of the first and second lithium ions. Taken together, the results suggest that the barrier to full lithium-ion insertion occurs during the first insertion stage and that an irreversible change occurs between the two stages. The results will help focus the future efforts of theorists and chemists in finding paths to improved performance.
Work performed at ALS Beamline 6.3.1.
L.W. Wangoh, S. Sallis, K.M. Wiaderek, Y.-C. Lin, B. Wen, N.F. Quackenbush, N.A. Chernova, J. Guo, L. Ma, T. Wu, T.-L. Lee, C. Schlueter, S. Ping Ong, K.W. Chapman, M.S. Whittingham, and L.F.J Piper, “Uniform second Li ion intercalation in solid state ϵ-LiVOPO4,” Appl. Phys. Lett. 109, 053904 (2016).