Dissolving carbon dioxide (CO2) in water produces carbonate systems that are key to many processes essential to life, from the buffer system that regulates pH levels in blood, to the carbon cycle that governs CO2 uptake by Earth’s oceans. A detailed understanding of such systems is complicated by the presence of an interface—a cell membrane or the ocean surface, for example—that the CO2 must cross and that could affect the behavior of the various carbonate species (molecules containing the carbonate ion, CO32–). At the ALS, researchers have used ambient-pressure x-ray photoemission spectroscopy (APXPS) to probe the relative concentrations of carbonates near an interface, finding a surprising reversal in the expected abundances of carbonate (CO32–) and bicarbonate (HCO3–) as a function of depth. The results raise important questions about what is really happening at interfacial regions, with relevance to topics ranging from carbon sequestration to biomedical research.
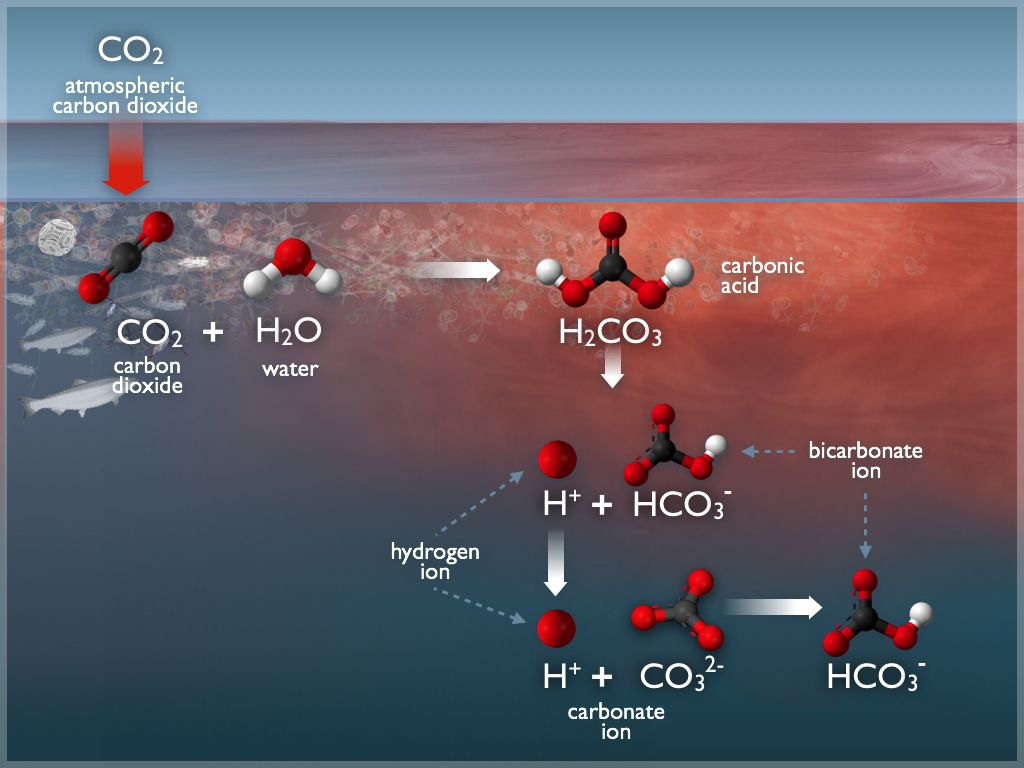
Earlier studies of carbonate systems had used x-ray absorption spectroscopy (XAS) at the ALS to probe the arrangement (hydration structure) of carbonate species and CO2 in water. The results showed how CO2 molecules dissolve in water to initiate the production of carbonic acid (H2CO3) and bicarbonate ions (HCO3–). However, XAS is mainly a bulk-sensitive technique. To gain access to important details occurring at interfaces, the researchers turned to APXPS, a surface-sensitive technique that allows atom-specific characterization of chemical states as well as depth profiling of the overall system. Because electrons emitted with higher energies have greater escape depths (effective attenuation lengths), they provide information about species deeper into the solution than electrons with lower energies. Thus, increases in the incident photon energy correlate to increases in the probe depth.
ALS Beamline 11.0.2 is one of the few beamlines in the world capable of this type of experiment on liquids at near-ambient pressures. Because APXPS involves the detection of electrons, any window used to separate the liquid sample from the detector would block electrons from reaching the detector. Instead, the researchers used a liquid microjet system that they developed, allowing them to directly inject a controlled stream of liquid into the chamber without breaking the vacuum.
A number of aqueous carbonate solutions were probed this way, including a 50:50 mixture of sodium carbonate (Na2CO3) with sodium bicarbonate (NaHCO3), and a 50:50 mixture of carbonic acid (H2CO3) with sodium bicarbonate (NaHCO3). In water, the sodium compounds dissolve into sodium (Na+), carbonate (CO32–), and bicarbonate (HCO3–) ions. Carbon 1s spectra of the carbonate species were collected using four incident-photon energies, roughly corresponding to depths of 2.0, 3.0, 4.5, and 6.0 nm. Gaussian fits to the data were used to deconvolute the contributions of the various carbonate species. Comparison of the resulting peak areas gave the relative concentrations of each species.
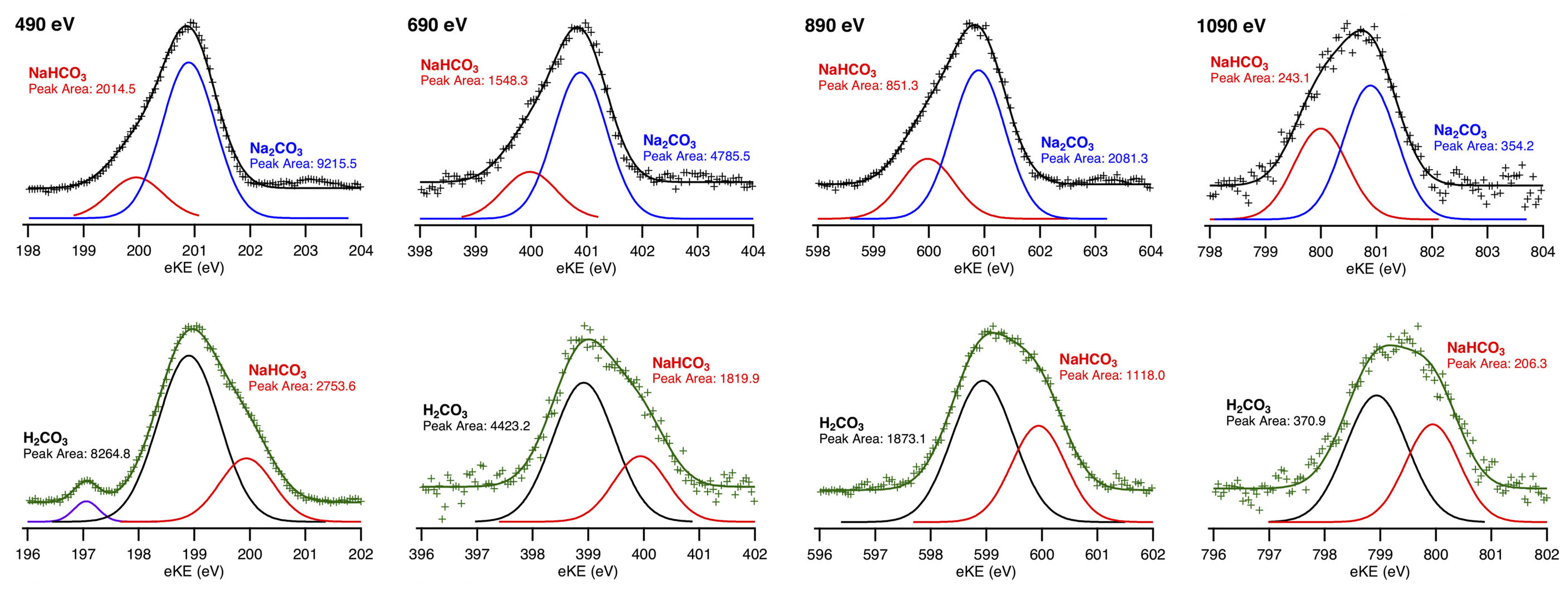
Analysis of the data showed that, as depth increases, the peak-area ratios approach unity, as would be expected in bulk regions of 50:50 mixtures. At shallower depths, however, both carbonic acid (H2CO3) and carbonate (CO32–) have higher concentrations than bicarbonate (HCO3–). This is not surprising for neutral H2CO3 relative to charged ions (because electrostatic effects tend to repel ions from interfaces). On the other hand, the enhancement of CO32– (doubly charged and strongly hydrated—i.e., attracting many water molecules) over HCO3– (singly charged and less strongly hydrated) is surprising and appears to conflict with recent models for interfacial ion adsorption.
The researchers suggest that the conflict could be resolved if CO32– adsorbs to the interface as an ion pair with sodium (i.e., Na+:CO32–), neutralizing some of the charge effects mentioned earlier. Further theoretical modeling will be needed to gain greater insight into this unexpected reversal in the depth profile of interfacial carbonate systems.

Contacts: Royce Lam, Rich Saykally
Research conducted by: R.K. Lam, J. Smith, A. Rizzuto, and R.J. Saykally (UC Berkeley and Berkeley Lab); O. Karslıoğlu (Berkeley Lab); and H. Bluhm (ALS and Berkeley Lab).
Research funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES). Operation of the ALS is supported by DOE BES.
Publication about this research: R.K. Lam, J. Smith, A. Rizzuto, O. Karslıoğlu, H. Bluhm, and R.J. Saykally, “Reversed interfacial fractionation of carbonate and bicarbonate evidenced by X-ray photoemission spectroscopy,” J. Chem. Phys. 146, 094703 (2017). doi: 10.1063/1.4977046
ALS SCIENCE HIGHLIGHT #352