High cholesterol affects more than 35 million Americans, putting them at high risk for heart disease and stroke. The most commonly prescribed cholesterol-lowering drugs, called statins, are effective but associated with a number of side effects, including muscle pain and digestive problems. Genentech has been working in collaboration with the ALS with the goal of identifying a better treatment mechanism that targets a cholesterol-regulating protein in the body known as PCSK9. Recent advances in understanding PCSK9’s structure have put them closer to that goal.
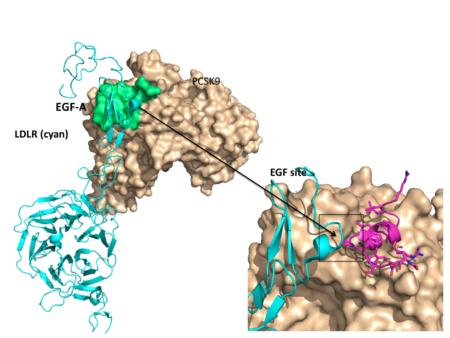
PCSK9 is a promising target for cholesterol-lowering therapeutics because it plays a unique role in the regulation of so-called “bad” LDL (low-density lipoprotein) cholesterol that deposits on the walls of the arteries. LDL cholesterol is cleared from circulation by the liver, by way of another protein called the LDL receptor. PCSK9 interferes with this process by binding to the LDL receptor, inhibiting its ability to remove LDL cholesterol particles from circulation and resulting in higher LDL cholesterol levels. Neutralizing PCSK9’s ability to bind to the LDL receptor on the surface of liver cells has become a new, and already successful, strategy to improve LDL receptor function and reduce LDL cholesterol levels in the blood.
The first PCSK9-based therapeutics, anti-PCSK9 antibodies, were approved by the U.S. Food and Drug Administration in 2015. However, they are expensive, costing about $14,000 per year per patient, and must be administered via syringe injection. On the other hand, a simple orally administered medicine (also known as a “small-molecule drug”) that targets PCSK9 could potentially be taken at home and would be much cheaper.
“What we’d like to have at some point is something that lowers cholesterol just by taking a simple pill,” says Mark Ultsch, principal research associate at Genentech. The key to achieving that goal is to identify a binding site on the PCSK9 protein where a drug molecule could attach. While most small-molecule drugs have well-defined binding pockets on the target of interest, PCSK9’s LDL receptor binding site (known as EGF(A), as the EGF(A) domain of the LDL receptor binds to this region) is relatively flat and ill suited for the development of small molecules.
Genentech has spent many years collecting hundreds of data sets using x-ray crystallography at the Berkeley Center for Structural Biology‘s Beamline 5.0.2 in search of a new way to disrupt the binding of the EGF(A) domain to PCSK9. They crystallize together PCSK9 proteins and inhibitory molecules and bring the resulting samples to the ALS to expose them to x-rays. The data derived allow Genentech to solve a three-dimensional structure, which gives them insights into the binding interaction.
The company’s recent findings, published in Nature Structural and Molecular Biology, report the discovery of a “cryptic” (hidden) binding site right next to the EGF(A) site on PCSK9. The cryptic site is a small groove that is normally occupied by a helical segment of PCSK9. However, the ALS data revealed that the helix displays conformational flexibility, or the ability to dynamically change shape, and can move out of the groove like a revolving doorway. Guided by this structural information, Genentech was able to engineer peptides that bind to the vacated grooves and then encroach on the EGF(A) binding site and inhibit receptor binding.
“There are still a lot of hurdles to overcome before we can develop an investigational medicine for the PCSK9 site,” says Ultsch. “We are just at the point now where we can see what type of scaffold we could use for that small molecule, but it would still be years and years down the road to be able to actually come up with new therapeutics.”
As an ALS user for eight years, Ultsch has seen a lot of improvements in ALS capabilities. “The software developed by ALS beamline scientists has been very helpful—it has greatly enhanced our ability to rapidly look at data sets and determine which ones were good,” says Ultsch. “And data collection hardware upgrades have enabled us to collect many more data sets.”
The ALS is set for another major advance for structural biology users in the next few years with the buildout of the Gemini beamline. With a leap in brightness and throughput capabilities, Gemini will further accelerate drug discovery efforts for companies such as Genentech by allowing even more difficult structural problems to be solved at a faster rate.
“Moving forward, we will continue to collaborate with the ALS,” Ultsch says. “We still have many more data sets to run, but we are really excited about where we’re going with this.”
Publication about this research: Y. Zhang, M. Ultsch, N. Skelton, S. Burdick, M. Beresini, W. Li, M. Kong-Beltran, A. Peterson, J. Quinn, C. Chiu, Y. Wu, S. Shia, P. Moran, P. Di Lello, C. Eigenbrot, and D. Kirchhofer, “Discovery of a cryptic peptide-binding site on PCSK9 and design of antagonists,” Nat. Struct. Mol. Biol. 24, 848 (2017). doi: 10.1038/nsmb.3453