SCIENTIFIC ACHIEVEMENT
Researchers using the Advanced Light Source (ALS) demonstrated that electrically induced mechanical strain can control the magnetic state of tiny magnets used to sort biological cells.
SIGNIFICANCE AND IMPACT
The work lays the foundation for a programmable, single-cell sorting platform to support a wide variety of biotechnology applications, including personalized cancer treatments.
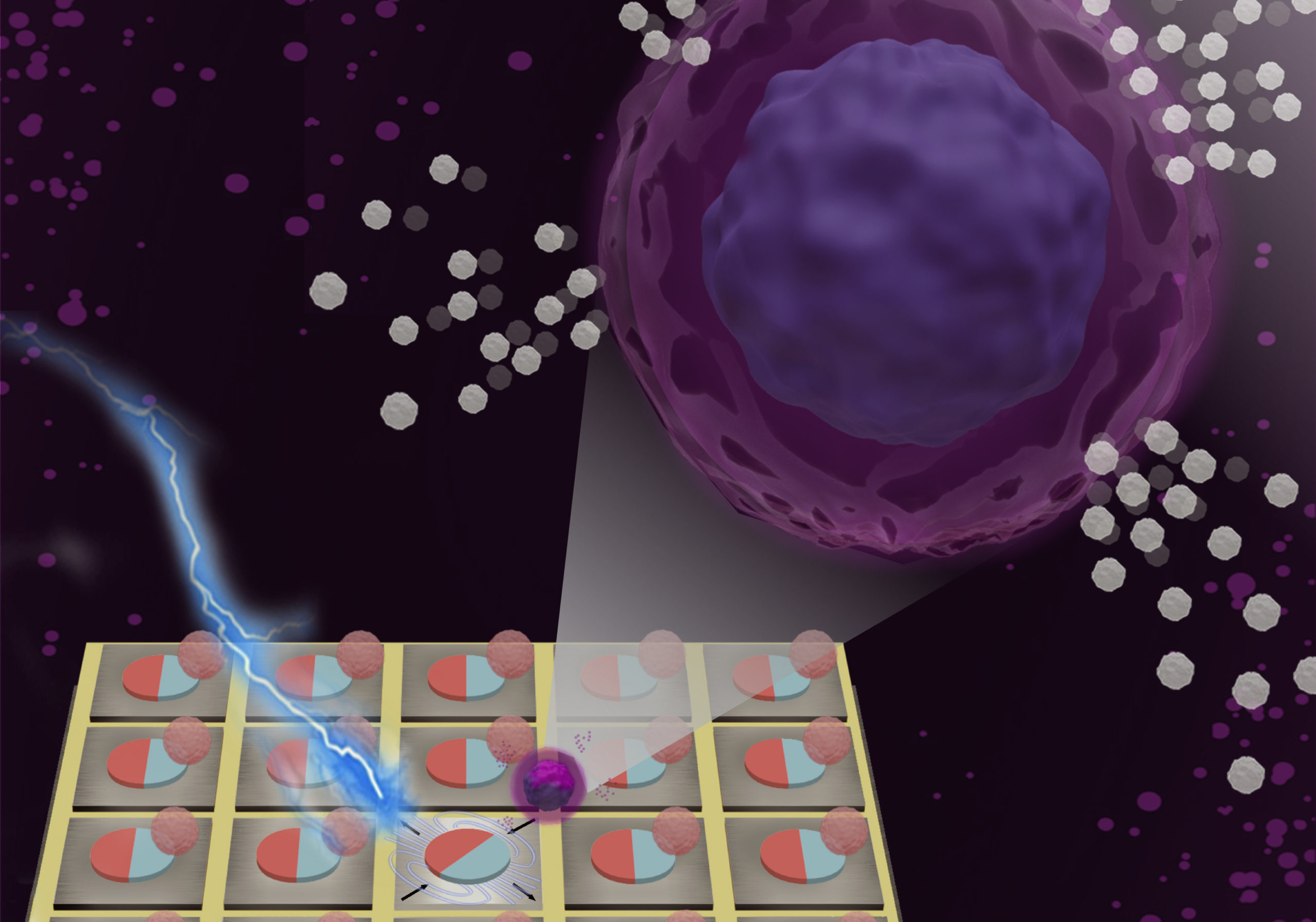
A need for selective cell-sorting
A promising personalized form of immunotherapy adapts a patient’s own T cells to fight certain types of cancer, such as leukemia and lymphoma. The process involves extracting T cells from the patient’s blood, re-engineering them to target cancer cells, and sorting them so that only the most effective are reproduced in the quantities needed for treatment.
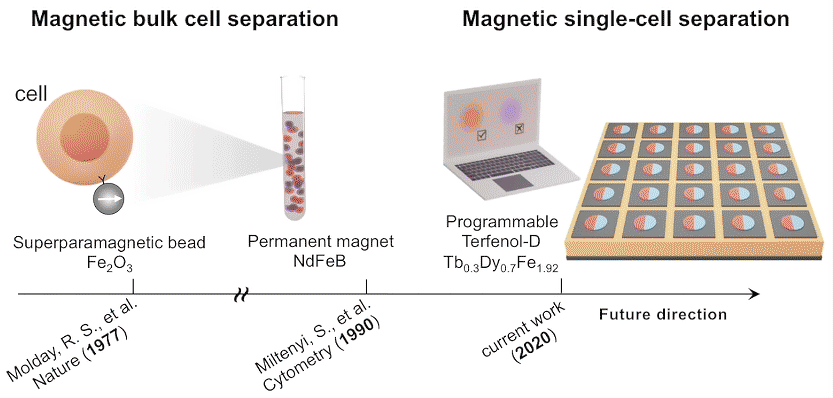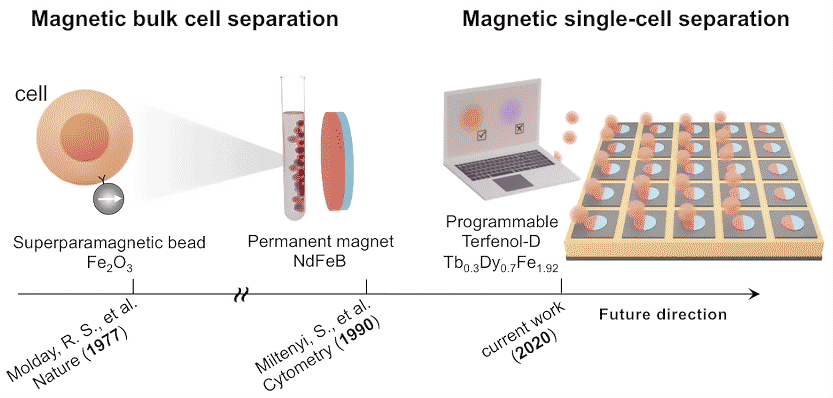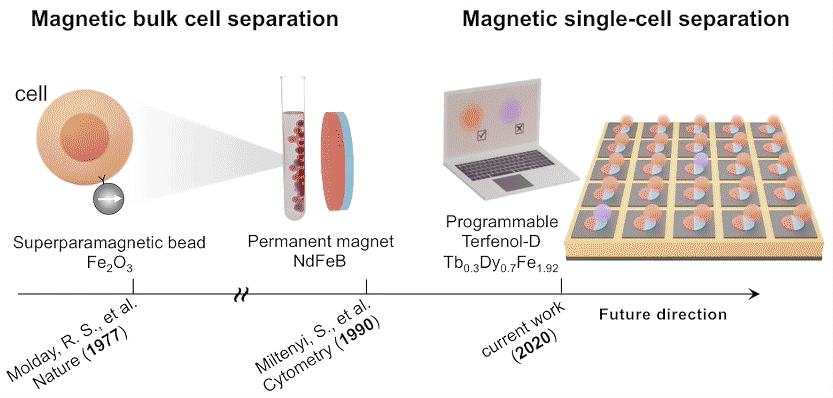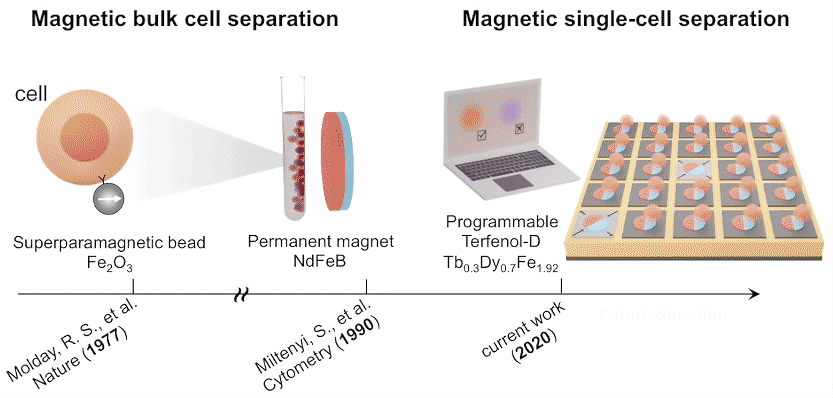
While high-throughput, bulk sorting can be done by magnetic tagging of cells, so far there has been no way to magnetically capture and release individual cells based on desired characteristics. Here, researchers used the ALS to characterize the properties of magnetic microstructures with electrically controllable magnetizations and demonstrated their ability to capture and release single cells on demand.
A strain-mediated switch
The researchers created microstructures using Terfenol-D, a metallic compound of terbium, dysprosium, and iron that’s highly magnetoelastic (i.e., its magnetization changes when compressed or stretched). Microdisks of Terfenol-D were sputter-deposited onto a substrate material (PMN-PT) that’s piezoelectric (i.e., expands or contracts in response to an applied electric field).
In concept, a voltage applied to a particular microdisk would act as a strain-mediated switch for the Terfenol-D magnetic state, allowing the programmed capture and release of cells tagged with magnetic beads, based on complex behaviors or functions (e.g., cell‐killing, secretion, motility).
Single-domain configuration
To characterize the magnetic properties of the Terfenol-D microdisks, the researchers used photoemission electron microscopy (PEEM) at ALS Beamline 11.0.1.1. X-ray magnetic circular dichroism (XMCD) experiments at Beamlines 4.0.2 and 6.3.1 provided supporting element-specific data. The ability to image the in-plane magnetization was crucial to obtaining direct evidence of the effective single-domain magnetic configuration of the Terfenol-D microdisks, even in the absence of an external magnetizing field.
The results showed that the Terfenol-D sustained the single-domain state to a scale comparable to that of a human cell (20 µm), an order of magnitude larger than other commonly studied magnetoelastic micromagnets. A large single domain produces a magnetic field strong enough to firmly capture magnetically tagged cells in microfluidic environments and are much more responsive to strain than multidomain structures.
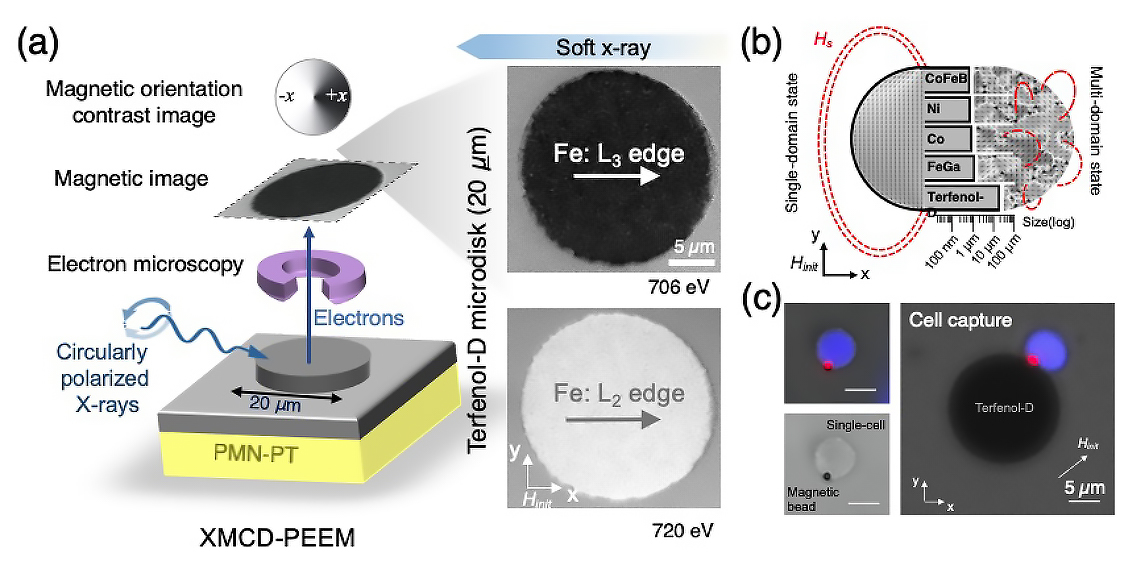
Proof of concept
To demonstrate how the platform works, the researchers flowed magnetic beads through a microfluidic channel on top of Terfenol-D microstructures, which readily captured the beads. When a voltage was applied to the substrate, induced strains in the substrate caused the Terfenol-D magnetization to rotate, reducing the attractive force between bead and disk. When the fluid drag force exceeded the magnetic force, the bead was released.
The researchers also tested the platform’s compatibility with the sorting of T cells based on the secretion of interleukin‐2 (IL‐2), a signaling molecule that regulates T cell proliferation and differentiation. Over time, magnetically trapped single T cells revealed two distinct sub‐populations, differentiated by a fluorescence signal associated with IL-2 accumulation.
In future experiments, the researchers expect to incorporate local surface electrode pads to generate a more diverse regional strain profile in the piezoelectric substrate. For now, these experiments lay the foundation for using large arrays of magnets to capture, observe, and selectively release cells with specific functions that may lead to improved therapeutic performance.
Contacts: Robert Candler, Maggie Xiao, and Reem Khojah
Researchers: R. Khojah, Z. Xiao, M.K. Panduranga, M. Bogumil, Y. Wang, G.P. Carman, R.N. Candler, and D. Di Carlo (UCLA); M. Goiriena-Goikoetxea and J. Bokor (Univ. of California, Berkeley); and R.V. Chopdekar (ALS).
Funding: National Science Foundation and Basque Government. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: R. Khojah, Z. Xiao, M.K. Panduranga, M. Bogumil, Y. Wang, M. Goiriena-Goikoetxea, R.V. Chopdekar, J. Bokor, G.P. Carman, R.N. Candler, and D. Di Carlo, “Single-Domain Multiferroic Array-Addressable Terfenol-D (SMArT) Micromagnets for Programmable Single-Cell Capture and Release,” Adv. Mater., doi:10.1002/adma.202006651 (2021).
ALS SCIENCE HIGHLIGHT #445