Researchers at the ALS have recently observed peptoid nanosheets—two-dimensional biomimetic materials with customizable properties—as they self-assemble at an oil–water interface. These nanosheets are an emerging class of two-dimensional nanomaterials that have the potential for use in a variety of applications ranging from molecular sensors to artificial enzymes. Because peptoids are highly designable polymers, nanosheets provide a general platform on which to display an enormous diversity of functionalities. This new development opens the door to designing peptoid nanosheets of increasing structural complexity and chemical functionality for a broad range of applications, including improved chemical sensors and separators, and safer, more effective drug delivery vehicles. The development was based the on collaborative research efforts of Berkeley Lab’s ALS and Molecular Foundry and the University of Oregon.
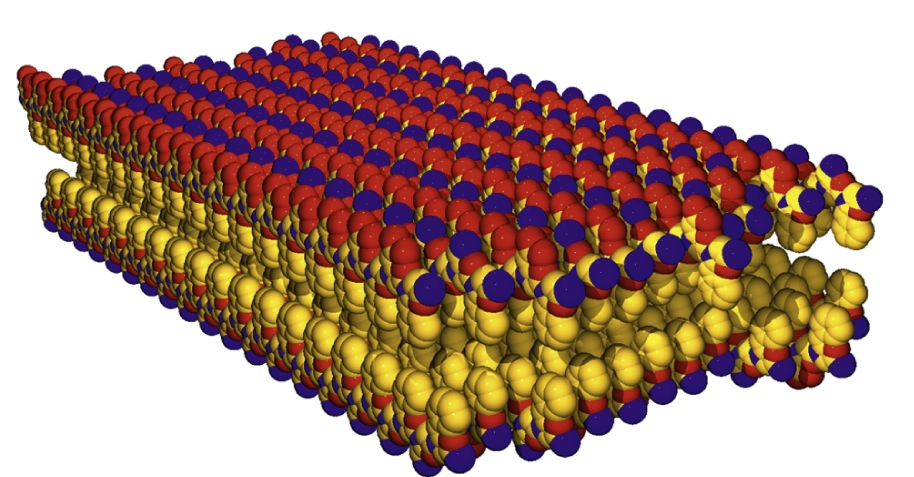
Biomimetic materials with customizable properties that self-assemble at an oil–water interface, peptoid nanosheets form from the self-assembly of a sequence-specific peptoid polymer at an air–water interface. Nanosheet formation occurs first through the assembly of a peptoid monolayer and subsequent compression into a bilayer structure. These bilayer materials span hundreds of micrometers in lateral dimensions and have the potential to be used in a variety of applications, such as in molecular sensors, artificial membranes, and as catalysts.
Recent advances in the design, synthesis, and characterization of two-dimensional nanomaterials with atomic precision have given rise to ultrathin materials with unprecedented functionality. Specifically, organic two-dimensional nanomaterials hold promise as biocompatible materials that can be chemically tailored and built from the bottom up, through the self-assembly of small-molecule, protein, or polymer building blocks. Polymer-based two-dimensional nanomaterials, in particular, hold promise as templates for bottom-up assembly of circuits, semiconductors, and organic–inorganic composite materials as well as high-surface-area membranes for filtration, catalysis, and sensing.
The oil–water interface also provides another opportunity for growth of these unique and highly ordered peptoid sheets. The monolayers formed at this interface have been found through surface spectroscopic measurements to be highly ordered and electrostatic interactions between the charged moieties, namely carboxylate and ammonium residues, of the peptoid are essential in the ability of these peptoids to form ordered nanosheets at the oil–water interface.
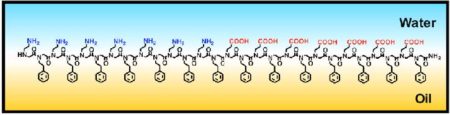
Supramolecular assembly at an oil–water interface is an effective way to produce 2D nanomaterials from peptoids because that interface helps pre-organize the peptoid chains to facilitate their self-interaction. This increased understanding of the peptoid assembly mechanism offers researchers the opportunity to scale up to produce large quantities, or scale down to screen many different nanosheets for novel functions.
The substitution of oil in place of air creates a raft of new opportunities for the engineering and production of peptoid nanosheets. For example, the oil phase could contain chemical reagents, serve to minimize evaporation of the aqueous phase, or enable microfluidic production. The production of peptoid nanosheets in microfluidic devices means that researchers should soon be able to make combinatorial libraries of different functionalized nanosheets and screen them on a very small scale. Berkeley Lab researchers are now investigating the addition of chemical reagents or cargo to the oil phase, and exploring their interactions with the peptoid monolayers that form during the nanosheet assembly process.
In the future, researchers may be able to produce nanosheets with drugs, dyes, nanoparticles, or other solutes trapped in the interior. These new nanosheets could have a host of interesting biomedical, mechanical, and optical properties. Expanding the mechanism of peptoid nanosheet formation to the oil–water interface and understanding the crucial role of electrostatic interactions between peptoid residues in nanosheet formation is essential for increasing the complexity and functionality of these nanomaterials.

Contact: Geraldine Richmond
Research conducted by: E.J. Robertson (University of Oregon), G.K. Olivier (Berkeley Lab), M. Qian (Berkeley Lab), C. Proulx (Berkeley Lab), R.N. Zuckermann (Berkeley Lab), and G.L. Richmond (University of Oregon).
Research funding: U.S. Department of Energy (DOE), Basic Energy Sciences (BES) and the Defense Threat Reduction Agency. Operation of the ALS is supported by the DOE BES.
Publication about this research: E.J. Robertson, G.K. Olivier, M. Qian, C. Proulx , R.N. Zuckermann, and G.L. Richmond, “Assembly and molecular order of two-dimensional peptoid nanosheets at the oil–water interface,” PNAS 3, 111 (2014).
ALS SCIENCE HIGHLIGHT #310